Rapid At-Home Tests for COVID-19 Finally Get FDA Approval. But People Still Need a Prescription.
Plus: Pennsylvania Supreme Court rejects Trump campaign complaint, new pandemic restrictions in lots of states, and more...
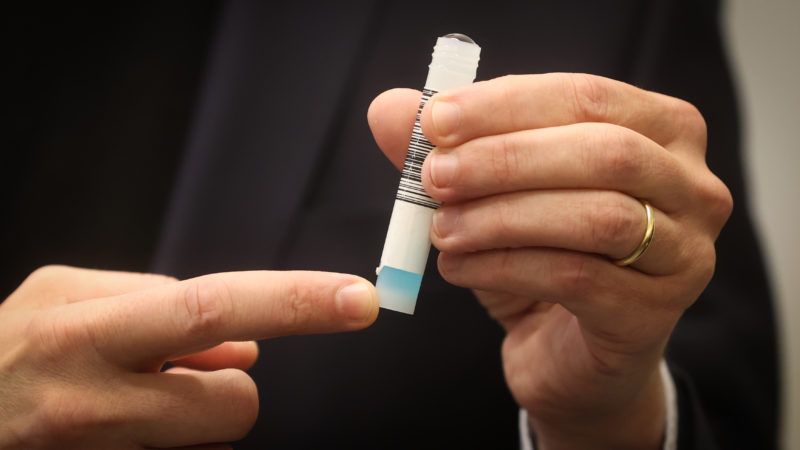
While coronavirus case counts keep rising and states keep imposing impractical new restrictions, this week also feels like a potential turning point in our pandemic experience. Initial COVID-19 vaccine trials from two different pharmaceutical companies—Pfizer and Moderna—have shown very promising results and the U.S. Food and Drug Administration (FDA) has finally approved an at-home rapid test for the virus. The single-use test, made by Lucira Health, can deliver results in 30 minutes.
Rapid results testing for COVID-19 is still scarce in a lot of places in the U.S., and at-home tests that don't need to be mailed into a lab for processing have been banned, so the Lucira test's approval is a big deal.
But at-home testing—and all its game-changing potential—is still being limited by government regulators, who will require people to get a prescription for the home test kit. Unless every single person gets prescribed the test via telemedicine, this ridiculous FDA rule has the potential to not only make at-home testing more difficult, less useful, and less widespread, but it could also put people at more risk.
Unfortunately, this sort of counterproductive meddling has been a recurring theme throughout the pandemic (and, well, always) for the FDA. The agency has so far been holding up at-home testing over paternalistic concerns that people would not be able to properly implement the tests or interpret results, reports the Associated Press:
More than two dozen companies have been racing for months to develop the first, rapid home-based test for COVID-19. However, the FDA outlined a number of study requirements for manufacturers.
These hurdles have less to do with COVID-19 specifically, and more to do with decades-long concerns about whether people without any medical training can accurately screen themselves and interpret the results.
The FDA has only ever approved one home test for an infectious disease—an HIV test. And even commonplace over-the-counter tests—such as home pregnancy kits—were subject to years of scrutiny before FDA allowed their use in the 1970s.
To the FDA, it is apparently preferable that a whole bunch of people don't get tested at all, or risk infection waiting for hours in a doctor's office or testing line, than for some small fraction of folks to somehow do their at-home tests wrong. Government math strikes again!
"To date, the FDA has authorized nearly 300 tests for coronavirus," notes A.P.:
The vast majority require a nasal swab performed by a health professional and must be processed at laboratories using high-tech equipment. A handful of tests allow people to collect their own sample at home—a nasal swab or saliva—that's then shipped to a lab, which usually means waiting days for results.
They also report that the Lucira at-home test kit "uses technology similar to genetic laboratory-based tests that are the standard tool for COVID-19 screening. That's different than most rapid tests currently used in the U.S., which look for viral proteins called antigens—not the virus itself."
ELECTION 2020
Pennsylvania's highest court rejects Trump campaign theory on election fraud. "The Pennsylvania Supreme Court threw out one of the Trump campaign's longest-running post-election complaints Tuesday, ruling that officials in Philadelphia did not violate state law by maintaining at least 15 feet of separation between observers and the workers counting ballots," reports NBC News. "The ruling is likely to undercut the Trump campaign's case in federal court, where Rudy Giuliani joined a hearing Tuesday afternoon to argue on behalf of President Donald Trump's effort to contest the election results in Pennsylvania."
Meanwhile, Trump has fired the guy who kept calling out his election interference lies. Christopher Krebs, director of the Department of Homeland Security's Cybersecurity and Infrastructure Security Agency, had been tweeting and updating his agency's website since the election with direct rebuttals to Republican claims about all sorts of voter fraud.
QUICK HITS
These wars have gone on for nearly two decades. What he's really saying is that he wants perpetual war for just-in-case reasons, knowing there's no real oversight from Congress or mandate from the American people. It's immoral and dangerous. https://t.co/VX48WmOQbt
— Justin Amash (@justinamash) November 18, 2020
• The Federal Reserve and Department of the Treasury are cooking up plans to put more American bank accounts under surveillance. If it's able to lower a "travel rule" threshold to $250, it will let the federal government access more people's financial data, explains Andrea O'Sullivan.
• I recently moved from Washington, D.C., to Cincinnati, Ohio. Reason Staff Editor Liz Wolfe moved from Austin to Brooklyn. We wrote back and forth about our differing experiences with the pandemic in these places.
• An update on California's pandemic orders, from the Los Angeles Times:
A California appellate court Tuesday stayed an injunction from a lower court that barred Gov. Gavin Newsom from issuing executive orders that create new law, the latest development in a legal challenge to the governor's response to the COVID-19 pandemic.
The stay granted by the 3rd District Court of Appeal prevents an order by a Sutter County judge from being enforced until the appeal from the governor's office can be considered by the higher court
• Ohio is closing all retail businesses between the hours of 10 p.m. and 5 a.m. (thereby forcing shoppers into a more concentrated time frame and more crowded stores).
• Pennsylvania also announced new rules:
If you missed today's media briefing where @SecretaryLevine discussed the four new #COVID-19 mitigation efforts in Pennsylvania, you can watch the video here:https://t.co/CQO55QZy42
— PA Department of Health (@PAHealthDept) November 17, 2020
• So did Colorado. And Oklahoma. And Montana. The biggest city in South Dakota. And Illinois.
• Sen. Chuck Grassley (R–Iowa), the second oldest person in the Senate, has COVID-19.
• Politicians don't like the First Amendment, a never-ending story…
It is unfathomable to me that the U.S. Congress is asking private businesses for "lists" of their users & FB's info on them. I'm not equating the two in terms of severity, but this is like Alabama trying to get the NAACP's membership lists. Reminder: Alabama lost that case.
— Chris Marchese (@ChrisMarchese9) November 17, 2020
• Rapper Lil Wayne was arrested for owning a gun:
Prosecutors are supposed to file charges to further public safety… not to put feathers in their own caps. https://t.co/ziD3PeFpOM
— Jennifer Bukowsky (@esqonfire) November 18, 2020
• Reason's Eric Boehm on why you shouldn't buy the debunked Dominion conspiracy theory.
• Twitter has introduced a feature called Fleets, similar to Instagram Stories, and real-time audio chatrooms (a la Clubhouse):
Twitter also announced #Spaces, a feature that will let users join virtual rooms where they can engage in real-time, audio conversations with others. pic.twitter.com/YpdRfW0PAi
— Pop Crave (@PopCrave) November 17, 2020
Editor's Note: As of February 29, 2024, commenting privileges on reason.com posts are limited to Reason Plus subscribers. Past commenters are grandfathered in for a temporary period. Subscribe here to preserve your ability to comment. Your Reason Plus subscription also gives you an ad-free version of reason.com, along with full access to the digital edition and archives of Reason magazine. We request that comments be civil and on-topic. We do not moderate or assume any responsibility for comments, which are owned by the readers who post them. Comments do not represent the views of reason.com or Reason Foundation. We reserve the right to delete any comment and ban commenters for any reason at any time. Comments may only be edited within 5 minutes of posting. Report abuses.
Please to post comments


https://twitter.com/ConradMBlack/status/1329036177467379713
"If you don't certify this flawed election I'm going to doxx you, call you a racist and hope BLM and Antifa 'peacefully protest' in front of your home after setting it ablaze."
Quote Tweet
Jack Posobiec Flag of United States
@JackPosobiec
· 10h
Democrat @NedStaebler threatening county officials when he didn’t get his way
He named her children and their school:
https://twitter.com/correctthemedia/status/1328969920357535744
BREAKING VIDEO:
The exact moment Democrat Abraham Aiyash threatened Monica Palmer's children on zoom. This extortion attempt directly influenced the decision to agree to certify the election fraud in #WayneCounty.
https://twitter.com/pnjaban/status/1328887187098660867
Thanks to
@TuckerCarlson
for shining a light on the incredible abuse, death threats, and economic terrorism being levied at
@realDonaldTrump
’s lawyers —
Quote Tweet
August Takala
@AugustTakala
· 12h
DHILLON: Every lawyer associated with Trump gets death threats — and incredible abuse from partners at major law firms. I've had abuse on my Facebook page from managing partners at law firms in San Francisco [who] represent actual criminals. (Harmeet Dhillon AKA @Pnjaban)
The little thuggish cowards in the little sissy Lincoln Project have balls associating themselves with Lincoln.
They're just a bunch of passive-aggressive losers.
I quit working at shoprite and now I make $65-85 per/hour. How? I'm working online! My work didn't exactly make me happy so I decided to take a chance on something new after 4 years it was so hard to quit my day job but now I couldn't be happier. Here’s what I do… WORK24HERE
MAGA 2020!!!
Google is by and by paying $27485 to $29658 consistently for taking a shot at the web from home. I have joined this action 2 gas months back and I have earned $31547 in my first month from this action. I can say my life is improved completely! Take a gander at it
what I do.........work92/7 online
[ PART TIME JOB FOR USA ] Making money online more than 15$ just by doing simple work from home. I have received $18376 last month. Its an easy and simple job to do and its earnings are much better than regularF office job and even a little child can do this and earns money. Everybody must try this job by just use the info
on this page.......CLICK DOLLER 666
remember kids, bullying is wrong -- unless we do it
https://www.linkedin.com/feed/update/urn:li:activity:6735056542040760320/
The other part is the election officials stated they voted yes solely in an agreement that there would be a post election audit. The governor has already said they will merely look into it, but there is no evidence of any issues... which means they won't.
Cannot fathom why so many do not buy this as a genuine election.
Cite for Whitmer saying "they will merely look into it"?
You are becoming quite pathetic.
You are not a good judge of that. Or of character in general.
Surely, you heard "they will merely look into it" somewhere, and didn't just make it up, so you could share your source.
No he’s right. You’re a pathetic bitch Dee.
That's how we know they're on the Right Side of History. The good guys always threaten children, right?
Hello.
Hello
Hello.
Hello!
Jello!
Nighty night sweetie.
Hell no!
Yo
It worked. The Zoom feed "Accidentally cut off" and the Republican members changed their votes.
Do you want to earn cash online from your living room, easily work with a laptop for a few hours a day, earn 550-650 euros a day and get paid every week by deciding on your working hours? it is all true and completely changed my life. Then try this. Here is More information.
I agree that the behavior of many of the people in the meeting was reprehensible, and it was wrong to doxx and call the Republicans racists.
However, when interviewed at the end of the meeting, William Hartmann said that the intimidation tactics did not influence his change of vote.
Did you write that shit with a straight face? WTF else would he say?
He could have said, “I changed my vote because I was intimidated by people yelling at me, and calling me a racist.” Or he could have said, “No comment.”
It is what HE said.
I guess under duress never occurred to you. He likely recanted because he wants his family to live.
Speculation on your part, that fits your partisan world view.
"Nice home and beautiful children, be a shame if something happened to them."
GFY
Yes, I'm sure when someone has a gun to their head, every word that comes out of their mouth from that point forward is the blessed truth.
There were people very angry at them, but no "gun to their head".
no, just a mob with molotov cocktails
Which were words, not literally Molotov cocktails.
Stop lying.
Too bad you're an incel or you may one day discover what you would do when a public official mentions your kids names and schools in a region of the country where multiple assaults, arson, etc is done for politics
LOL, now I'm an incel.
oh well, as long as the murder and arson threats didn't change his mind
I love how even democrats are making fun of the 'peaceful protest' designation.
But at-home testing—and all its game-changing potential—is still being limited by government regulators...
Wait, is the FDA heroes today or not? I'm losing track.
No explanation of how it could change the game.
Fuck those FDA assholes! They are totally in the pockets of the AMA and doctors! Regulatory capture! I am surprised that one doesn't need a dentist's prescription for toothbrushes. If toothbrushes were invented today, you WOULD need a prescription!
A while back, Narcan was by-prescription per FDA idiots! States bypassed them and allowed state-wide prescriptions for Narcan! Sample = https://www.naloxonesaves.org/for-pharmacists/faqs/
Statewide Standing Order FAQs
We need to do the same with the testing kits, to bypass FDA assholes! I wrote a letter to my State Rep with the same suggestion per the "lung flute", and never even got a response! I am too old and tired to bother any more... My outrage glands are worn out! You young whupper-Schnuppers who have energy left, PLEASE run this suggestion in to your State Rep!
"I wrote a letter to my State Rep with the same suggestion per the “lung flute”, and never even got a response! I am too old and tired to bother any more… My outrage glands are worn out! You young whupper-Schnuppers who have energy left, PLEASE run this suggestion in to your State Rep!"
State Reps have no legal authority or influence on FDA.
The US House and Senate have oversight of FDA.
Unfortunately for public health, consumer choice and market competition, Big Medicine, Big Pharma and Big Healthcare have massive lobbying and have given huge political $$$$$$ to key House and Senate Committee members with oversight of FDA.
In sum, organized medicine, drug companies and healthcare service providers want to profit from the covid pandemic, and the easiest way for them to make money is by eliminating or limiting competition.
End the FDA is just as important and end the Fed
Are these the same government regulators whom we've been assured are going to grant approval to two new vaccines in just a matter of a couple of weeks and by next month there's going to be millions of vaccine doses available? The same regulators who keep vaguely reassuring us that they're going to make sure the vaccines are safe and effective first when we all know the normal process for making sure a new drug is safe and effective takes about 6 years? Are we supposed to believe that their normal process is just a matter of foot-dragging or that their expedited process is not in fact quite as thorough at guaranteeing the safety and efficacy of the vaccine? It seems to me you can't have it both ways, either the expedited process isn't as good as the normal process or the normal process is unnecessarily lengthy - or somebody's lying about how quickly these vaccines are going to move to market.
It’ll all be cleared up once Trump concedes.
https://www.chron.com/news/nation-world/article/Romney-defends-5-sons-lack-of-military-service-1572191.php
BETTENDORF, Iowa — Republican presidential hopeful Mitt Romney on Wednesday defended his five sons' decision not to enlist in the military, saying they're showing their support for the country by "helping me get elected."
No one should have to defend a decision to not join the military. We're not at war and face no real threats.
Not to mention the story is 9 years old.
"...officials in Philadelphia did not violate state law by maintaining at least 15 feet of separation between observers and the workers counting ballots," reports NBC News.
The Rona trumps all.
You said Trump!
Prepare to have all your accounts deleted!
https://twitter.com/mtracey/status/1328756153250160640
Ah, so the need to uncover the truth about the supposedly world-historic Mueller investigation suddenly "fades away" when it no longer has short-term political relevance. The catastrophism Dems used to foment panic and fear about Trump was 99.9% bullshit
Quote Tweet
Greg Stohr
@GregStohr
· 21h
NEW: House Democrats ask Supreme Court to postpone Dec. 2 arguments on sealed materials from Mueller probe, pointing to Biden's election, advent of new Congress. Good chance case now simply fades away.
It's all coming out when Biden takes charge of the executive branch. It's Christmas for the next 4 years. You're getting coal.
Gonna give you kudos for at least being honest with your biases unlike Jeff and co.
I'll take you off the Gulag list.
We are all getting coal.
Except coal causes global warming, and is no longer allowed by your overlords.
Only if you burn it. 19th Century depolorables at least got to use their coal to keep warm. 21st Century deplorables get a lump of coal to keep their pile of government paperwork from blowing away.
I thought that your lord and savior, Barack H. wan't keen on coal.
I am now making more than 350 dollars per day by working online from home without investing any moneyy..Join this link posting job now and start earning without investing or selling anything.
Follow Instructions Here....... Home Profit System
No way in hell the democrats give out the evil coal.
That’s just silly. They will likely be doing a lot of taking, but no giving.
https://twitter.com/BillFOXLA/status/1328932610169561090
EXCLUSIVE: We've obtained photos of Governor Gavin Newsom at the Napa dinner party he's in hot water over. The photos call into question just how outdoors the dinner was. A witness who took photos tells us his group was so loud, the sliding doors had to be closed. 10pm on
@FOXLA
No kidding.
They KNOW masks and social distancing is bull shit.
The aristocrats really just don't give a fuck about being caught in their hypocrisy.
I guess when the journalists work for you you're pretty much invincible.
Yeah this. I’m old enough to remember when politicians were embarrassed, at the very least, when they were caught being hypocrites and breaking the rules. Now, it’s almost a daily occurrence when left wing politicians are caught doing this shit, and it’s nbd.
being hypocrites
It should be noted that the issue isn't hypocrisy, the issue is oppression. If Newsome just went on stage and said "Everybody should wear masks and social distance." and then didn't wear a mask or distance himself, NBD. The problem is Newsome issues orders and passes laws shuttering businesses, crippling private and social organizations, and disrupting religious and family gatherings and then goes on to break those laws himself. I don't give a shit that he's a hypocrite. What really invokes the 'urge to kill' is that he's an oppressive hypocrite.
Yep
this
Newsom apologized for it so its all okay now. when Dems apologize thats all thats needed even if someone dies from covid at that party.
It'd be nice if they all died, though I'd prefer it not be from covid
If Poe was still alive would he rewrite the Mask of the Red Death?
https://twitter.com/iowahawkblog/status/1328828259547623424
Gotta bookmark this excuse to use the next time I accidentally crash into a liquor store
The cool thing about being an elected official is when you totally fuck up you can call it a "teaching moment" and then start lecturing everybody about how we all need to do better
Gov. Gavin Newsom apologizes for attending party at French Laundry: "We're all human. We all fall short sometimes."
Only some us go to jail.
https://freebeacon.com/2020-election/warnock-americans-cant-serve-god-and-the-military/
Warnock: ‘Nobody Can Serve God and the Military’
'Choose ye this day who you will serve,' said the Democrat
Intersectionality allows everyone to be vile bigots except working class whites who just happen to be the Davos crowds biggest bugbear. No wonder it's now the dominant philosophy of the establishment.
It's how impossibly rich white men make war on the poor ones in a grab for power. Black bigots like Warnock are just disposable tools in the effort.
Bullshit.
The democrats DO NOT allow choices.
(unless a dead baby will result)
Judas Iscariot was the first progressive:
"'Why wasn't this perfume sold and the money given to the poor? It was worth a year's wages.' He did not say this because he cared about the poor but because he was a thief; as keeper of the money bag, he used to help himself to what was put into it."
https://pjmedia.com/news-and-politics/tyler-o-neil/2020/11/17/watch-ted-cruz-skewers-jack-dorsey-on-twitters-suppression-of-ny-post-story-n1153731
“You claim [The New York Post story] was ‘hacked materials’ and yet you didn’t block the distribution of the New York Times story that alleged to talk about President Trump’s tax returns, even though a federal statute makes it a crime to distribute someone’s tax returns without their consent. You didn’t block any of that discussion, did you?”
Dorsey admitted that Twitter did not block that discussion. Instead, he split hairs to try to justify the double standard. “In the New York Times case, we interpreted it as reporting about the hacked materials not distribution of the hacked materials,” he said.
https://pjmedia.com/news-and-politics/tyler-o-neil/2020/11/17/hawley-claims-to-have-clear-evidence-that-facebook-google-twitter-plot-censorship-together-n1154015
Facebook confirmed to PJ Media that the company does work with Twitter and Google to fight terrorism but repeated Zuckerberg’s insistence that it does not coordinate with other tech companies on content moderation.
“You’re coordinating together to control information,” Hawley said to Zuckerberg. “In recent days, my office was contacted by a Facebook whistleblower, a former employee of the company, with direct knowledge of the company’s content moderation practices. And I want to start by talking about an internal platform called Tasks that Facebook uses to coordinate projects including censorship.”
“The Tasks platform allows Facebook employees to communicate about projects they’re working on together. That includes Facebook’s censorship teams, including the so-called community wellbeing team, the integrity team, and the hate speech engineering team, who all use the Tasks platform to discuss which individuals or hashtags or websites to ban,” the senator claimed.
Zuckerberg admitted that Facebook employees use the Tasks system “for people coordinating all kinds of work across the company.” It seems to be something of a company-wide to-do list.
Hawley presented a screenshot of Tasks, mentioning “referenced to election integrity.”
“What particularly intrigued me is that the platform reflects censorship input from Google and Twitter as well,” the senator claimed. “So Facebook, as I understand it, Facebook censorship teams communicate with their counterparts at Twitter and Google and then enter those companies’ suggestions for censorship onto the Tasks platform so that Facebook can then follow-up with them and then coordinate their censorship efforts.”
They’re private companies, so it’s ok if they collude, and spy on users.
Reason libertarianism means libertarian philosophy should only be pursued by the American federal government.
Everyone else is free to book-burn, censor and cancel their fellow man.
Soft fascism is cool because the agreements with government is just a wink wink.
After seeing that Democrat senator (forgetting his name) ask Dorsey why he’s not censoring more speech that doesn’t agree with the liberal agenda, it’s not even just that anymore.
https://pjmedia.com/news-and-politics/matt-margolis/2020/11/17/flashback-univ-of-michigan-computer-scientist-warned-about-voting-machine-vulnerabilities-in-2018-n1153707
In April 2018, the Times published an opinion video featuring J. Alex Halderman, a University of Michigan computer scientist, demonstrating how easy it would rig a voting machine to a group of students.
In the video, Halderman demonstrates how voting machines are “dangerous” and “obsolete” by holding a mock election with University of Michigan students. Halderman had previously testified before Congress, warning that computerized voting is “vulnerable to sabotage” and “cyberattacks that could change votes.”
“I’m here to tell you that the electronic voting machines Americans got to solve the problem of voting integrity, they turned out to be an awful idea,” Halderman says in the video. “That’s because people like me can hack them, all too easily.”
Halderman set up an election with various computer voting machines, asking students to vote between the University of Michigan and their arch-rival, Ohio State University.
Ohio State ultimately “won” the election because Halderman had already hacked the machine to silently switch the votes.
https://hotair.com/archives/allahpundit/2016/12/27/yougov-poll-52-of-democrats-believe-russia-tampered-with-the-vote-totals-to-get-trump-elected-president/
YouGov Poll: 52% Of Democrats Believe Russia Tampered With The Vote Totals To Get Trump Elected President
https://www.weforum.org/great-reset/
THE CONTEXT
The Covid-19 crisis, and the political, economic and social disruptions it has caused, is fundamentally changing the traditional context for decision-making. The inconsistencies, inadequacies and contradictions of multiple systems –from health and financial to energy and education – are more exposed than ever amidst a global context of concern for lives, livelihoods and the planet. Leaders find themselves at a historic crossroads, managing short-term pressures against medium- and long-term uncertainties.
THE OPPORTUNITY
As we enter a unique window of opportunity to shape the recovery, this initiative will offer insights to help inform all those determining the future state of global relations, the direction of national economies, the priorities of societies, the nature of business models and the management of a global commons. Drawing from the vision and vast expertise of the leaders engaged across the Forum’s communities, the Great Reset initiative has a set of dimensions to build a new social contract that honours the dignity of every human being.
.. new social contract that honours the dignity of every human being.
As long as you think the right things.
to help inform all those determining the future state of global relations, the direction of national economies, the priorities of societies, the nature of business models and the management of a global commons
You mean "everybody", right? Because that's how democracy works, everybody has a voice in determining "the future state of global relations, the direction of national economies, the priorities of societies, the nature of business models and the management of a global commons". It's not like Top Men or some sort of Elite Political Class get to decide all these things for themselves and then simply impose their decisions on everybody else - that would make it some sort of medieval feudal system where the nobility hold all the rights and privileges and the serfs simply do as they're told. And we know that's certainly not what they're talking about.
Great Reset
New World Order
Fourth Reich
Whatever you call it, it's a bad idea.
https://twitter.com/wretchardthecat/status/1328840112780632064
The establishment is conflicted over whether to regard China as a strategic partner or potential antagonist. Most of Washington wants to do business with them. Some even want to emulate them. The most telling sign is there's no "Chinese collusion" narrative.
China is an antagonist.
Let's go for a pint.
Oh wait. China isn't allowing it. /Chinese puppet pulls strings with smirk.
China is an antagonist.
This whole virus thing seems to have worked out pretty well for China. Few cases (that they report, anyway), a China friendly US president, and a world economy in shambles. I am sure it is just a coincidence.
Not to mention they gobbled up Hong Kong while nobody was looking.
I brought that up yesterday...
Most of Washington wants to do business with them
The Uniparty's been selling out the country to the Chinks since Kissinger convinced Nixon to normalize relations with them, so this isn't a surprise.
...this week also feels like a potential turning point in our pandemic experience.
The walls are closing in on the Rona. Bombshell.
https://twitter.com/seanmdav/status/1329068654349185025
A massive Danish study on mask usage found no statistically significant difference in coronavirus infection rates between mask-wearers and non-mask-wearers. In fact, according to the data, mask usage may actually increase the likelihood of infection.
Yeah. But this merely agrees with 40 years of research. We have models developed this year that assume masks work, so the models prove the assumption.
WEAR THE MASK! Are you trying to kill jeffy? Because this is how you kill jeffy!
If jeffy were gone, his mom would end up eating all the cookies she buys for him, and then you have killed jeffy's mom too, you Brutalist!
OK, so is there a down side?
Nope
Tulpa won't have as many accounts to clone?
It took the Danish researchers four months to get this study published in a journal (whose publishing decisions have also become polarized on many controversial public health issues)
https://www.acpjournals.org/doi/10.7326/M20-6817
Had this study been published four months ago, some/many countries, states, cities and corporations wouldn't have relied upon mask mandates as their key strategy to reduce new cases of covid, which failed in many US states and in most European countries.
Then again, perhaps twitter, Facebook and Google will decide to block and/or otherwise censor news stories about this study (or perhaps block the study or even the journal).
Back in September, the CDC published a study that found 71% of people who tested positive for covid (at walk in clinics and hospitals) reported ALWAYS wearing a mask in public during the preceding two weeks, while another 14% reported OFTEN wearing masks.
Similarly, 74% of the control group (who didn't test positive for covid) also reported ALWAYS wearing masks in public.
https://www.cdc.gov/mmwr/volumes/69/wr/mm6936a5.htm?s_cid=mm6936a5_w
Unfortunately, this CDC study never highlighted its most important finding (it was buried in one sentence and at the bottom of the data tables), and CDC never notified the news media about this finding (because it contradicted CDC's policy urging everyone to wear a mask).
yes, breathing your own exhaust reduces your immunity.
it's why the heat radiators in NYC buildings are so hot -- they crank out enough heat to keep the room warm with the windows open, so you can breathe fresh air to combat the Spanish Flu.
Ohio is closing all retail businesses between the hours of 10 p.m. and 5 a.m. (thereby forcing shoppers into a more concentrated time frame and more crowded stores).
Yes but in the daylight (well, for maybe 9 of those hours) so you can see who's wearing a mask and who isn't.
The plague isn't contagious in daylight.
makes you wonder what the curfews are really for
What exactly does home testing do? It does nothing.
If you have been exposed to COVID, you need to isolate for 14 days. I am doing this right now. If I could test myself every day, *I would still need to isolate*. The problem with COVID is you test negative in the morning, and by that night you have symptoms. Several of the people in the same position as me, who have all been isolating, had exactly this problem. They go in for the Rapid PCR test in the morning, go home, and then have COVID the next day. If they weren't isolating, at some point they'd probably have been shedding the virus all over town.
In fact, the demand that the FDA authorize Rapid tests has had the exact OPPOSITE effect of containing the virus. If you search online for "Rapid COVID Test", 80% of the urgent care clinics will sell you an ANTIGEN test. These tests have a nearly 50% false negative rate. So you have all these people getting exposed to the virus, going and dropping $200 on an antigen test, and then using the negative result as proof that they can go out and party.
I'm glad that the FDA is moving more quickly to authorize these tests. However, the absolute shit-show that is the market for rapid testing is proof positive that all these journalists hyping up medical solutions to a complicated problem have no idea what they are talking about.
Honey?
Yes?
The Covid test says I'm negative.
Great!
But it also says I'm pregnant!
Your boyfriend is gonna be ecstatic!
I'm done being polite to these emotional terrorists and work from home paper pushing pussies.
Same here. So ready to let a Karen-masker have it.
The home COVID test that was approved by the FDA is NOT an antigen test, and is much more accurate. However, if it's going to require a prescription, then it won't do anything. In order to be a game changer, it needs to be simple, fast, accurate, cheap and accessible. If everyone had a bunch of these tests, you could test in the morning, and if you were negative, go to work, if positive, isolate. Or, if you were going to a party, take it an hour or so before you are ready to leave for the party. If it's accurate enough, a negative test will mean you aren't contagious. But you'd have to test every day, or at least every day you are going to go somewhere. So it would have to be cheap enough that people could afford to buy a month's supply at a time. Of course, if you test positive, you would probably then need to get a lab test to confirm, isolating until you get the results, then further isolation if it's positive. You could start testing daily again after 10 days or so of isolation, until you get a negative result, then get a lab test to confirm.
If we've gotten to a point that we have to constantly test ourselves multiple times a day for a fucking coronavirus, of which there are already multiple strains floating around and mutating constantly and have been for decades, we might as well pack it in.
exactly when will the testing end. Never. And i leterally mean never they are using this to change society.
It's just the flu.
"The home COVID test that was approved by the FDA is NOT an antigen test, and is much more accurate. However, if it’s going to require a prescription, then it won’t do anything. In order to be a game changer, it needs to be simple, fast, accurate, cheap and accessible."
I don't care how cheap and accessible this test is. There is NO WAY in hell that people are going to shove a fucking cotton swab into their noses and scrape their sinuses multiple fucking times a day. "Alright, time to head to the wedding. Got my dress on and the kids have their suits. Ok EVERYONE LINE UP TO HAVE YOUR DAILY BRAIN SKEWERING!"
That isn't going to happen. At best, people are going to just lightly swab the nose, not sinuses and get false negatives. More likely, they are going to test themselves once and declare victory, just as they have been doing with Antigen tests for the past 3 months.
I choose to not test and go about my life as if they hadn't dressed up the fucking flu as some sort of new, unique threat.
The problem with COVID is you test negative in the morning, and by that night you have symptoms. Several of the people in the same position as me, who have all been isolating, had exactly this problem. They go in for the Rapid PCR test in the morning, go home, and then have COVID the next day. If they weren’t isolating, at some point they’d probably have been shedding the virus all over town.
And this is only in the 'false negative' section of the decision tree. I've been in close contact, no masks, no air circulation, closed cabin with at least 2 people who supposedly contracted COVID before interacting with me and (they) subsequently developed symptoms/tested positive. Two weeks in isolation, no symptoms. And this is by no means an exceptional outcome. If I test positive, was I ever communicable? If I test negative, were they?
Moreover, it's not a one-off decision tree (which is how the test is being validated), but with the stupidity of lockdowns, it's a Markov Chain. One of my kids was also in close contact with one of 'the infected', the others weren't. Two weeks with myself and kids, no symptoms. If we all test negative, WTF does that even mean?
It means that there is literally nothing abnormal about this year's coronavirus itself.
The reaction to it is a hoax, and the most massive crime against humanity ever perpetrated.
We've been there before. Remember when Ronald and Nancy Reagan got drug tested, and encouraged everybody to get tested? What was that supposed to tell you? Was anybody in doubt as to whether they were using a drug? If it was a matter of showing the result to someone else, why would you do so if the result was positive?
Ga is up to 5600 new votes, mistakenly not added, in just 3 counties.
https://www.foxnews.com/politics/georgia-election-audit-uncounted-ballots-two-counties
Georgia has 159 counties. This is just the votes they forgot to tally. This isn't even a discussion of votes that were fraudulent.
Some people didn’t do some things.
It sure is amazing how all those mistakes and accidents always go one way. Must be some unknown law of physics in action.
We know you stood in line for 4 hours to cast this vote, but we didn't even count it. Oh well.
Oh come on now! There was no fraud!
https://twitter.com/Doc_0/status/1329059160307212289
If you treat voting like filling out the comment card at a restaurant with low marks because your burger was undercooked, don't be surprised that you end up with garbage government run by a greedy and inept ruling class. You allowed voting to become a joke. The joke's on you.
What he's really saying is that he wants perpetual war for just-in-case reasons, knowing there's no real oversight from Congress or mandate from the American people.
Look, do you want them to break with Trump or not?
Which team is throwing an epic temper tantrum when they lose an election they thought was theirs by right?
Which team is deliberately undermining the integrity of the election process in order to cast doubt on an election that they lost?
Which team is going to court, over and over again, to try to use unelected judges to thwart the will of the voters?
Which team is spreading all kinds of wild baseless conspiracy theories on the Internet in order to throw sand in the air and cast doubt on the election that they lost?
Which team is acting like spoiled children when things don't go their way?
Answer:
Team Blue, 2004
Team Red, 2008
Team Blue, 2016
Team Red, 2020
All of you: STFU and let the adults run things for a while.
Um. No. Other than the backlash for the election, the biggest cunts are the left-wing peaceful protestors and Democrats who put America through hell with outright lies since 2016.
Democrats who put America through hell with outright lies since 2016.
What do you think Republicans are doing now? With their insane conspiracy theories and whatnot?
The two teams are more similar than you care to realize. They both have a bunch of brain-dead zombies that follow their Dear Leader and devour whatever narrative comes from their preferred media bubble.
When a special prosecutor is convened to investigate you will have a slightly smaller false equivalence. But keep pretending the neutrality.
Oh look, you have the semblance of an admission by Team Red Robot Jesse that Republicans are in fact having an epic temper tantrum and putting the country through hell just because they lost an election that they thought was theirs by right.
Answer his point or fuck off, Jeff.
He’ll do neither, unfortunately. He’ll probably say something dishonest though.
Geez, you are dumb.
The fraud is so blatant that you have to be insane (or a participant) to deny it.
Example of fraud that is so blatant?
Fuck you.
You lemmings keep asking for evidence, and people keep providing it.
Then you assholes close your eyes and ears while repeating the mantra "there's no evidence".
Then hivemind comes back and asks for evidence again.
You need to die.
The “people keep providing it” part has not been happening.
Yes, it has, and you're a dishonest piece of trash who needs to die ASAP.
Yet you cannot come up with an example.
Don't bother Nardz. The second you do this sickening piece of shit will immediately ghost the thread, and then be squawking tomorrow about never getting a cite.
Just in this thread alone there are already about twenty cites on this very fucking subject that this clown has seen but is purposefully playing ignorant about. If he went up or down he'd immediately see them.
He's such a trash interlocutor.
Yeah because 3 weeks and 3 years are the same thing.
Yet it totes the same. Go fly an anvil.
I wasn't old enough to follow politics in 2004, but the Democratic Party has every reason to be upset about 2016. Russia's attack on our election was comparable to Pearl Harbor and 9 / 11.
#TrumpRussia
#LibertariansForGettingToughWithRussia
"I wasn’t old enough to follow politics in 2004"
*Chef's kiss*
Yes, but you're a lefty shit who only pretends independent allegiance when your team wins. You've admitted this.
Fuck off Jesse. You are one of the immature children who cannot see beyond tribal bullshit.
You are in a tribe jeff. Youre one of the lefties who pretends to be neutral but only attacks one team. You even called yourself a fucking proud globalist you lefty shir. Want to discuss immaturity? Lose some weight so your health doesn't have to protected by other people. Take responsibility for your own failures.
Lol, I had forgotten that Jeff admitted to being fat.
Morbidly obese.
Oh look it's Troll Mac here to troll. Why don't you put forward some idea of your own instead of just trolling everyone else. You won't because you are a coward, because that would open you up for criticism of your ideas. You are afraid to put forth an original idea of your own because you are insecure of your own abilities. Much more fun to just troll and troll, right? It takes actual work to actually imagine a constructive solution to a problem. You don't have it in you to put in that work. You are no better than Tulpa at this point.
Definitely hit a nerve with fatty here.
Why don't you actually use your entire brain for once instead of shilling for one team. You have no original ideas. You are just a parody of a right-wing Twitter account at this point. You swallow every right-wing demagogic trick and you have no shame in just being used like a whore by the Team Red grifters. Here is a clue, "proud globalist" is NOT equivalent to "lefty", but in Trumpworld, I suppose anything that is not Trump is "lefty". That is how Trump has corrupted your brain and has destroyed your few last remaining brain cells. I hope you actually grow up and put to use some of that education that you presumably got at some point in your life and put down your social media feed. You are pathetic.
You're not fooling anyone, Jeff.
You've done nothing but constantly lie here and shill for the DNC. Time and time and time again you've been caught sockpuppeting and lying your ass off.
Nobody believes you anymore. It's time to fuck off a find a home where people don't yet know how full of shit you are.
Mental health and physical health are related. Lose some weight and you’ll feel better overall.
LOL Can you name a few "proud globalists" who are right wingers?
Rhetorical question. There is no such thing.
It’s Lying Jeffy. He likes to say dishonest things.
I can name "proud globalists" who are not lefty. They are called libertarians.
No. They are lefty shits who lie about being libertarian. Globalism requires an authoritarian aspect to disallow the formation of smaller units.
Everything globalist push is closer to communism than liberty dumbfuck.
* “proud globalist” is NOT equivalent to “lefty”*
now pull the other leg
Immaturity is not having the balls to call yourself what you are, and pretending your ideology is other than it is because that gratifies your ego.
Yes, chemjeff is extremely immature.
Says the guy who comes here on a daily basis with murder fantasies of his opponents.
It's not murder if it's war.
Your existence is an active threat to my, and others', freedom.
STFU and let the adults run things for a while
Yeah, we've been letting "the adults" run things for about 100 years and look where it's gotten us.
You know, the other day I was reading an article lamenting the growing (Trumpian) strain of anti-intellectualism, for that is exactly what populism is - anti-intellectualism. And I wondered, what's wrong with anti-intellectualism? Isn't the opposite of intellectualism simply "common sense"? I mean, nobody calls a heart surgeon or a chemist or a civil engineer or a plumber an "intellectual" despite the fact that you have to be pretty knowledgeable to do these things. No, "intellectuals" are people who engage strictly in the rarefied air of "theory" rather than the common mud of practical knowledge.
Specifically, intellectuals engage in theories of human nature and human action. Which, to develop complex theories of human nature and human action, you first have to assume that humans have no free will, that they are not greedy, self-interested, morally-equivocating, sneaky, rotten little bastards, that they can be as easily manipulated as a sockful of ball bearings, they don't respond in unpredictable ways to incentives and disincentives, and that they act "rationally" in accordance to your definition of rationally rather than yours.
Which is why the War on Drugs hasn't worked out quite the way "the experts" thought it would, why every goddamn government project comes in late and over budget, why every government program to "fix" a problem seems to make the problem worse, why things cost more and do less than what was promised, why socialism always seems to entail killing the shit out of a lot of people who refuse to get with the program and act the way you think they should act rather than acting the way they want. Because the "experts" all have great theories about how things should work, but not a goddamn one of them has any real-world experience with how things really work or a lick of common sense that should warn them that things don't work the way you think they should. Notice there's not one fucker at the headquarters of the Department of Labor or the President's Council of Economic Advisors who successfully started up and operated a successful business, but they all got advanced degrees in economics and business management and shit like that. Not a goddamn one of them knows how to do shit, but they're all experts in telling you how shit ought to be done.
Spoken like a true intellectual.
"Yeah, we’ve been letting 'the adults' run things for about 100 years and look where it’s gotten us."
This.
I'm curious, Jeff, which adults should be running things now?
Uncle Joe was an 'adult'. He got things done. The trains to Siberia always ran on time.
I spend a lot of time navel-gazing with the best of them about the theories of knowledge, and the best ways to organize things, and the like. So you can probably say I err on the side of Intellectualism. But I also don't want to tell you how to live your damn life.
People who bemoan the advent of "Anti-Intellectualism" are actually bemoaning a population that rejects Intellectuals running their lives. Jeff is a perfect example above. He wants "The Adults" running things. Whether it is the "more mature" or "better educated" or whatever, we see that this isn't about intellectualism, so much as Elitism. The elite should be running things, and fuck you plebe for objecting.
Redneck populists would be at home shooting their guns, if Intellectuals and Elites weren't spending their every waking day trying to manage those populists into some utopian state. And I would probably be arguing the finer points of various philosophies with friends over wine, if it weren't for the Intellectuals and Elites. It is those people and their desire to enforce their worldview- they are the ones ensuring that any debate is about how they can ensure that everyone has the option to undergo transition surgery at 5 years old. And so I reject them, and the Media calls that anti-intellectualism.
Feh.
And I wondered, what’s wrong with anti-intellectualism?
People used to talk up the movie Idiocracy like it was some defining prognostication on the future of the human race. I would point out that the central premise was a contrived plot device. That, in order for it to be true or a prognostication, the brightest minds humanity has to offer would have to be incapable of conceiving of the things that jack rabbits and cockroaches do with only the dimmest glimmer of awareness.
The intervening years have reflected well on jack rabbits and cockroaches.
Boom. This. This applies to idealists as well.
What do you mean by 'undermining the integrity of the election process?'
Current or past examples work.
Genuinely curious.
You are right. All correct thinking people know that Joe Biden is such an inspiring leader that the record breaking vote totals are totally not odd on their face, and doubly so only in swing states. But don't worry if we remove all checks and simply declare everything is on the up and up that will make it so to all correct thinking people, the others we'll put on lists for educating again.
Good point, but 2016 was the worst, a 4 year temper tantrum. At least in 2020 the loser is trying to follow the legal process before the vote counts are final.
Christopher Krebs, director of the Department of Homeland Security's Cybersecurity and Infrastructure Security Agency, had been tweeting and updating his agency's website since the election with direct rebuttals to Republican claims about all sorts of voter fraud.
And when restaurants open back up they're still not going to sit a former Trump admin official, so it bought you nothing!
We wrote back and forth about our differing experiences with the pandemic in these places.
It was interesting.
It was a boring example of elites out of touch with everything but the advertising click count.
The stay granted by the 3rd District Court of Appeal prevents an order by a Sutter County judge from being enforced until the appeal from the governor's office can be considered by the higher court
I can never follow these sentences.
The crooked state supreme court told the crooked county court to go to hell.
Here, one of the Robococks pushing their little shitty measures not rooted in science, admitted there's not much more they can do (well, there is. The theory is called 'circuit breaking' which is essentially Martial Law. What's stupid about this theory peddled by asshole virologists is that it will only break the cases but not make the virus go away. So why fricken do it then? Why further damage people's lives to cut cases by 65%? That is, if it works at all. Sure, you can put us all in cages for six weeks but the virus is still out there, no?) since people are starting to ignore the decrees.
And rightfully so. But this mask thing is out of control. They added shields to this nonsense. Yet, cases keep shooting up and they keep threatening with lockdowns. At some point the scared sheep have to realize these masks don't work. Period.
The other thing that must stop is perniciously blaming people for the spread. This is just plain dehumanizing. People are exhausted and morale is low.
But enjoy Biden. His plan is more masks and lockdowns.
Joe Biden. GTFOH. Trump was 100% correct: LIVE YOUR LIFE.
"The other thing that must stop is perniciously blaming people for the spread. "
I have been through the schools reopening here in California. I have watched my 8 year old daughter spend her entire day at school with a face-mask, sitting at a desk with glass shields all around it. Each day she goes to school with her smile hidden, unable to enjoy 3rd grade because the "experts" have turned it into joyless isolation.
Now they are saying we need to lockdown harder. And they have the temerity to tell my daughter it is because she didn't flagellate herself hard enough? That is complete horseshit.
At this point, the public needs to understand that one of two things will happen: 1) Masking and lockdowns don't actually stop the spread of the virus or 2) It is physically impossible to enforce Masking and Lockdowns to the extent that it achieves a reduction of the virus.
I am willing to accept that it is #2, given that I have seen how many tyrant leaders are happy to flout the regulations for their own purposes. But whether it is #1 or #2 doesn't matter. The Lockdowns don't work. The Mask Mandates don't work. Screaming harder at people to "MASK HARDER!!!!!" has been tried for 5 months, and hasn't worked (especially given the hypocrisy of the leaders). So it is time to MoveOn to something different.
It's psychological child abuse.
There are kids who have natural radiant smiles that are being hidden. Remember when they used to say it's infectious? This is healthy. I'm at a loss for words that this mask thing has gotten to this point. The province of Saskatchewan just mandated. We're waaaaaaaaaaaaaaaayyyyyyyyyyy past the point of this remotely working. How is it that public officials haven't looked at the HARD DATA and see it DOES. NOT. WORK?
And that we mask children up is just beyond the pale.
I literally would slap an official if they said this was appropriate to my face.
That's how much I've had it with this anti-science, inhumane obscenity.
But it starts with people and parents. STOP BEING AFRAID and PUSH BACK.
Even in deep blue states that have called lockdowns, thousands of people are still out and about, driving around and going about their business. Because they've now seen that, even when they follow the rules, it ultimately didn't make a difference and cases spiked anyway. Small businesses that did the same and had no indication that they were the cause of these spikes are told by governors, "sorry, you're 'non-essential' and you have to close down. Doesn't matter that these spikes aren't your fault.'"
These assholes don't really care about you or the businesses getting cornholed. They're flailing in the face of the utter failure of their "experts" to predict this, and expecting the federal government to bail them out due to their failure. And why wouldn't they? After all, it was just 12 short years ago that the feds did the same for the banks who committed securities fraud and sent the economy into a tailspin as a result. Why wouldn't the governors expect their own bailout after deliberately putting a bullet in their economy's head?
People started ignoring the lockdowns as soon as the mass protests in the streets weren't condemned a superspreader events, but celebrated as social change. They realized it was a sham.
Yup.
Because it's the fucking flu.
Like I said yesterday, if the governor of your own fucking state won't follow the rules he set out for others that would supposedly prevent community spread, then there's no reason to believe those rules actually work, and he's just getting his jollies off of tormenting you, your family, and everyone else in California.
Same goes for every other governor that's jerking themselves off to all these rules that they passed, which utterly, completely failed to stop the annual spike in respiratory illness that was inevitable, anyway.
https://twitter.com/Doc_0/status/1329054684024365057
You can't have it both ways. You can't insist voting is such a sacred privilege that it overrides every other consideration, while also treating the votes themselves as worthless junk - protected and counted far less assiduously than every state tracks its weekly lottery tickets.
Which team again wanted to stop the count when they were ahead with in-person voting, and wanted to continue the count when they were behind with in-person voting? Who is treating votes like worthless junk now, which ought to be overturned by unelected judges?
Why are you so against and auditable election? The only team that stopped counting were democrats in the contested counties. All the gop did was want to stop until election watchers were in place you lefty shit.
Election watchers *were* in place. Why did Trump and others misrepresent that fact.
Your insistence on posting deliberately obtuse comments is tiresome.
Why do you insist on repeating partisan lies?
Cite?
https://www.usatoday.com/story/news/factcheck/2020/11/17/fact-check-2020-election-whats-true-and-whats-false/6266732002/
From the (long) article:
On poll workers, challengers and electors
Fact check: Viral video shows Pennsylvania poll workers fixing damaged ballots
County officials confirmed that workers were fixing damaged ballots and the video has been manipulated to crop out bipartisan observers who witnessed the process. The damaged ballots have been preserved, and Pennsylvania's Election Code states damaged ballots must be duplicated.
Fact check: Videos showing crowd locked out of Detroit TCF Center with windows obstructed are missing context
Viral videos depict crowds of challengers stuck outside Detroit's TCF Center as election workers count ballots inside behind obstructed windows. Statements from officials and witnesses clarify that Republican challengers were not more frequently denied entry, and the TCF Center's windows were covered because election workers felt intimidated.
No, cite the partisan lie I repeated.
What are they even counting? Legit votes? Who really knows?
Jeff and the rest of the leftist apologists at Reason, commenters and writers alike, don't care as long as we get away from calling out the media for their bias and back to the civility of calling voters dog faced pony boys.
Sure, right. Juice implies some nebulous "they" is counting illegitimate votes. That's just paranoid partisan belief.
Cite?
https://reason.com/2020/11/18/rapid-at-home-tests-for-covid-19-finally-get-fda-approval-but-people-still-need-a-prescription/#comment-8592464
"What are THEY [emphasis mine] even counting?"
Prosecutors are supposed to file charges to further public safety… not to put feathers in their own caps.
LOL
https://twitter.com/Doc_0/status/1329071970059599875
None of the restrictions on speech and other freedoms imposed in the name of fighting the coronavirus will go away when the pandemic subsides. They will be repurposed to serve other items on the elite agenda, which we'll be told are every bit as serious as Covid-19 was.
"Just as Americans came together and made great sacrifices to defeat Covid-19, so we must now come together and save our beloved planet from the pandemic of climate change."
We'll also be told that we must remain submissive to certain coronavirus practices forever, because the next pandemic could be right around the corner. Gigantic sums of money will be appropriated - and then wasted or stolen - with such warnings ringing in our ears.
Yep.
Cops, and I mean the whole take you away and punish you system, have to say no. They have to refuse to enforce these demands. Once a few jurisdictions here or there tell a mayor or governor to fuck off, more will follow.
There needs to be consequences for the politicians, technocrats, and media pushing this bs.
Mortal consequences.
It is the only thing that will stop them.
Yup. Sooner or later, somebody with the authority needs to walk into the governor's mansion with handcuffs.
That's ultimately what the Paris Accords are for--it's not to "stop climate change," it's to act as a wealth transfer from the First World to the Third World, with banks like Goldman Sachs and Chase acting as middlemen to make millions, if not billions, when they get their cut.
Any agreement that classifies India and China as "developing nations" after several decades of First World-level economies and the worst pollution on the planet, is a joke to begin with.
First to third world is just how they dress it up.
It's wealth transfer from the middle and working classes to the global socialists and their cronies.
Capitalism transfers wealth to the poor, governments steal wealth for a select few
Like airport security after 9/11.
Like section 230.
https://twitter.com/AlexBerenson/status/1329042770732969985
It may be worth noting (though it won’t be) that only 10 severe cases of #Covid were found in the trial’s 44,000 participants. It would be nice to know how long the average participant was followed to get some sense of a one-year risk ratio for severe disease...
Participants were followed for one month (and the treatment group was given masks to wear for one month). But it took four months for the completed study to get published.
The full text of the study is at
https://www.acpjournals.org/doi/10.7326/M20-6817
Reason's Eric Boehm on why you shouldn't buy the debunked Dominion conspiracy theory.
Gonna guess because it was debunked.
I better not find out that Koch Industries owns Dominion, buck-o.
With a name like Dominion, they probably own everything and everyone.
Well, declared debunked by correct thinking journolisters at any rate. Ignore their previous concerns over the exact same thing because the totals came out their way this one last time.
Official sources said it was debunked.
Meanwhile, Trump has fired the guy who kept calling out his election interference lies.
ENB's still in the same spot on the Coup-o-meter but she's working it hard.
Reason V.3.0 Coup-o-meter:
Step 1: Get mail-in voting approved to guarantee a non-credible election result.
Step 2: Have the Democrat-controlled media immediately proclaim Biden as winner.
Step 3: Use media acolytes to make it “true” that Trump lost. Frame any challenges to the narrative as the ragings of a dictator, clinging to power. <— TODAY'S ROUNDUP
Step 4: If the courts and congress find Trump’s electoral victory is legitimate, claim that it’s not. Activate rioters.
Step 5: Remove Trump by non-Constitutional means. Frame it as saving the nation.
Step 6: 'Build Back Better' baby. Make sure that those uppity surfs pay.
Just look at this:
"Christopher Krebs, director of the Department of Homeland Security's Cybersecurity and Infrastructure Security Agency"
First off CISA is a standalone United States federal agency. It works in Association with the DHS, not under.
Secondly Krebs entire argument was that the election was secure because of the procedures in place, not because they went and checked to see if they were violated. He knows it's dishonest, the agency associates who signed the letter know it's dishonest.
What they were doing is deliberately creating a misleading sound bite for DNC media operatives like ENB to wave around.
He shouldn't be fired, he should be arrested. These are the actions of a coup plotter.
Shout in the streets about how your boss is wrong.
See how long you keep your job.
Oh, come on. Trump's firing of the election security expert was blatant.
So, the guy with the keys covering his ass by saying, " I am absolutely sure every door was locked, I guarantee nobody got in," is to be believed without ever looking at the security tapes when the CEO says the inventory counts don't make sense? Or do you fire his ass?
And Trump carefully considered that he had to say? Nope, he impulsively fired him, via tweet. Perhaps while sitting on the toilet.
Nope, he impulsively fired him, via tweet. Perhaps while sitting on the toilet.
C'mon, you gotta admit that would be awesome.
"Yu're [uh! plop!] fired!"
Nope. I do not think there is even one drop of "awesome" in having an impulsive President.
I do not think there is even one drop of “awesome”
Got it. WK suffers from constipation and can't imagine the pure joy that firing a useless technocrat while on the shitter would bring.
If it helps, just consider it multi-tasking, buddy. And stop eating so much cheese.
White knight is made of nothing but resentment and misanthropy.
It is not a man, it is literal garbage.
So, if Kirkland is a jar of gall bladders of failed dictators, WK is equivalent to a jar of appendixes of discredited government scientists (think Public Health Service, working with the Tuskegee Institute). Where the Rev is bilious and repulsive, WK is well meaning, but blind to inevitable outcomes of policy. Got it.
As an aside, that would make jeffy equivalent to a jar of Robespierre's ass fat with enough skin still attached to display his Liberte, Egalite, Fraternite tramp stamp.
Enjoy your senile crook President and his corrupt prosecutor backup.
I do not think there is even one drop of “awesome” in having an impulsive President.
So you think we should wait a few more months, maybe even a year, to see how the COVID thing shakes out before taking any action then?
Impulsive? Been over a week since the media first said he did.
Not that many placebo cases, when you define a case as needing actual symptoms:
https://twitter.com/jacyanthis/status/1329058376471490562
Pfizer Covid-19 vaccine data is out!
Placebo Vaccine
20,000 people 20,000 people
162 cases 8 cases
9 severe cases 1 severe case
So.... covid isn't that serious from the study data.
I'm sure the people who volunteer for these trials are extremely low risk.
They had some old farts, can't (easily) find out how many, but a few. And the sickies were in the placebo group.
Twitter has introduced a feature-
Stop right there.
President Trump went through with his order to draw troops down in Afghanistan to 2,500--in harmony with the full withdrawal agreement he signed with the Taliban back in February and per his constitutional authority as Commander-in-Chief.
"After 19 years in Afghanistan, it’s high time to bring our troops home!"
----Rand Paul
https://parler.com/profile/Randpaul/posts
If I'm reading Parler right, his post received 11,902 up votes.
Am I reading Parler right?
If Parler is nothing but right-wing nuts, at least 11,902 of them seem to be the kind of right-wing nuts who don't support forever wars.
yeah, but ENB said they were racists
Welcome home. Here's your mask, stay away from your relatives and Church. Good work and thanks for your service.
That up-vote total has gone up almost a thousand votes since I posted this comment.
Lin Wood: “This election was a fraud. Donald Trump won I believe a 70% plus landslide election in the nation. He probably won over 400 electoral votes.”
100%. No way Joe fricken Biden - Lord Supreme of Losers - won with 306 EV. That's just insulting the intelligence of people.
Joe Biden. GTFOH.
PS: Joe Biden is a crook.
What's really horrifying is the media's enthusiastic participation in destroying American democracy.
Apparently the vaccines by Pfizer and Moderna are a brand new kind using mRNA technology. I definitely think that's great for vulnerable people, even though we don't know what the long term effects will be. If they want to try it, they should be able to. My concern is that most of the people in power act like we are ALL equally vulnerable, so of course they are going to try to force us to take this vaccine using completely new tech. And the conspiracies on me can't help but wonder how this relates to the Great Reset planned.
I have two little kids who are otherwise totally up to date on their vaccines, but this vaccine kind of scares me.
*conspiracist in me, stupid auto correct
Yeh there are too many questions and valid ones.
question as now after moderna said 95% effective after Pfizer said 90% has now come out to claim 95% effective. a little one upmanship makes me worry about the efficacy of the product.
I will feel better about it when fauci injects his grandkids on live tv.
I can't help but notice that Fauci keeps talking about how you don't have to worry about this vaccine because the government's going to make sure it's safe and effective, and nobody ever questions him on exactly how long he expects it to take for the government to make sure it's safe and effective.
And if you know Fauci's history with refusing to allow the use of AIDS treatments that Europe had approved under the "well, I'm dying of AIDS, how much worse could the treatment be?" principle, you know that that fucker will stand on your neck to keep you from getting at something that may help you if he isn't convinced it will help you as much as he thinks it ought to.
Fauci is an unprincipled weasel.
/stares out at the woodchipper.
you know that that fucker will stand on your neck to keep you from getting at something that may help you if he isn’t convinced it will help you as much as he thinks it ought to
"I can't breathe..."
"Stop resisting! I am trying to keep you from hurting yourself!"
I still wouldn't trust him or even believe he was using the vaccine.
People with various hyperactive autoimmune disorders should probably be hesitant--plenty of those hyperactive autoimmune disorders still aren't well understood.
You have a debilitating condition because your autoimmune system is fighting something that isn't there, so you beef up your immune system with a new type of vaccine to fight something that isn't there?
It may well be that taking the vaccine is warranted for such people. My understanding is that cytokine storm is often the cause of mortality in COVID-19 patients, and it's my understanding that people with hyperactive autoimmune disorders are more susceptible to that than others--because they already have a hyperactive autoimmune system.
If the vaccine is effective in warding off the virus, it may be worth the risk of exacerbating the systems of a hyperactive autoimmune disorder--not that there's any reason yet to assume that the vaccine necessarily will exacerbate those symptoms. It's more like it might make sense to wait and see what happens after those vaccines have circulated in the general population.
The reason Microsoft's new features and fixes sometimes break existing programs is because Microsoft can't account for every possible hardware and software configuration out there before it releases updates into the wild. When they release these vaccines, they're weighing the presumed risks to people with your particular autoimmune disorder into consideration, but they're balancing that against their legitimate interest in stopping pandemic among billions of people who don't have your disorder, too.
Anyway, point is I agree with what you're saying. We should all be free to make choices for ourselves using our own qualitative criteria, and, as always, we should be thinking of ourselves, too. There isn't anything about the appeal to authority fallacy that suddenly stops being true during a pandemic, and with the progressives in power, who are all about forcing individuals to make sacrifices for what they see as the common good, the time to assert our right to choose not to be vaccinated is right now.
If the vaccine only kills 246,000 people in 10 months it would still be a win, right?
Even the progressives might concede that it won't be in your interests if you're one of those 246,000!
And, like I said, there are bad things that can happen that aren't as bad as dying but still bad.
We should all be free to make choices for ourselves--so long as we don't violate anyone's rights.
Progressives have killed hundreds of millions over the last century.
They won't bat an eyelash at killing another 250k.
I've already said that, while it's great they could get a vaccine out this quickly, I'll hold off on getting one until we see what happens to the first guinea pigs when this goes into mass commercial distribution.
they will give it to the high risk individuals who may die of other cause but will we know if it was other causes or a failure of the Vaccine. I will hold out for the second tier persons to get it
https://twitter.com/DavidShafer/status/1329062200737148932
One of our monitors discovered a 9,626 vote error in the DeKalb County hand count. One batch was labeled 10,707 for Biden and 13 for Trump - an improbable margin even by DeKalb standards. The actual count for the batch was 1,081 for Biden and 13 for Trump.
Biden’s margin of victory in this batch of votes (99.9%) bested Bashar al-Assad’s 2007 margin (97.6%) and Raul Castro’s 2008 margin (99.4%). It matched Kim Jong-il’s 2009 margin (99.9%).
Look. Everyone knows who the local mobster is and who the town whore is.
Same here. There was fraud. Everyone knows it. Pretending like thee wasn't is being as deliberately naive and stupid as willingly wearing a mask thinking it works.
Problem is the entire system is against Trump. He was a tiny circle of loyalists and lawyers. He's not likely to overcome this one last fraud they hurled at him. From what I can see, very hard to over turn these things. Powell's Kraken better be one heckuva bombshell.
It's a shame but if they get away with it, that's it. Integrity in the system is broached.
So, did this take Biden's 13k state lead and reduce it by 9500? Between this and all the ballots they're finding, GA may end up for Trump.
Agreed, they should be checking the Senate votes too.
I cannot wait for WI, MI and PA recounts. AZ too. And NV. Even if Biden still wins, integrity of our voting system needs to be upheld.
Start making extra income Earns upto $550 to $750 per day by working just online... Read More.
What do you call it when you make fraudulent claims of fraud? Is that metafraud?
What's it called if you say the claims of fraud are fraudulent, but it turns out they weren't?
They should just mail out testing kits to every household, like they did with ballots. Then count the results as accurately as ballots. Everyone gets covid and votes for Biden.
Gonna need to lockdown more because the CFR is 275%
"In rare rebuke of Trump, NATO chief warns against troop cuts in Afghanistan "
[...]
"Afghanistan could once again become a haven for international terrorist organizations that seek to harm Western countries if foreign forces leave too abruptly, the head of NATO said Tuesday in a rare rebuke of U.S. policy, following reports that the Trump administration would withdraw thousands of troops from the country.
[...]
Trump has repeatedly threatened to pull troops out of Afghanistan, part of a broader promise to roll back open-ended U.S. involvement in foreign conflicts. His proposals have won cautious praise from critics on the left who accuse the U.S. military of ongoing overreach abroad. "
Yeah, Obo, and the hag are really behind ending wars and we only need 50 more years of US taxpayer money to make sure, right?
The article in the Chron pointed out that the real problem has to do with gas money; the US provides all the transport.
Fuck 'em; buy new boots and walk. I'm tired of paying taxes so frogs can wave their white-cross-on-white-field battle flags.
Time to end NATO.
It achieved its purpose on February 1, 1992.
If it is so important the rest of the NATO members can fill in the gap for the next decade or two.
Rapper Lil Wayne was arrested for owning a gun
And not just any gun:
“Onboard, in a Coach bag belonging to the musician, the agents reported finding a gold-plated .45-caliber Glock handgun with a pearl grip. According to a warrant, Lil Wayne said he had received the gun as a Father’s Day gift.”
https://www.nytimes.com/2020/11/17/arts/music/lil-wayne-gun-charge.amp.html
I gotta get me one of those.
The gun sounds like an abomination even by decorative standards. Maybe if it were a heavily modified Glock, but I wouldn't even want a solid gold casting of a Glock, much less a .45 cal. one. I'd rather have the "kids" that get you a pearl-handled, .45 cal. Glock than the Glock itself.
On the other hand, multiple searches have come up empty. This glock is kind of hard to picture. Every search I do just comes back with 1911s.
You really expect a New York Times reporter to understand the difference between a Glock and a 1911?
The only date they will acknowledge is 1619, the year no slaves were brought to Virginia.
I mean, that was my suspicion, but I don't know how to search it out otherwise.
The linked Miami Herald, article, which is way better than the NYT, calls it a gold plated clock according to the police. Apparently, Wayne's attorney is hopeful that the supreme court would hear this as a 2nd Amendment case. And rule favorably based on its current make up.
*glock not clock
This is the POS I'm picturing.
Something like this...
Or this might be worth keeping.
I'm a little befuddled about the .45 cal. Glock as a decorative piece. Either somebody really loves Lil Wayne and knows his taste *very* well or they just walked into the gun shop and said "I need a Glock, gold plated with a pearl handle by Father's Day." and the guy behind the counter understood that money/practicality was no object.
A plastic gun with a pearl grip?
A gold-plated plastic gun?
Lil Wayne said he had received the gun as a Father’s Day gift.”
Reginae Carter is, no shit, the best daughter ever.
>> gold-plated .45-caliber Glock handgun with a pearl grip.
I'm Castor Troy!
"Oooo-weee, you good lookin'!"
Thats what he gets for supporting Trump
thought this too.
here's an idea how bout I do what I want and not get tested because fuck these terrorists. Also can anyone of you useless fucking media whores ask a single relevent question of these gov like why these fucking retards why they are basing their lock-down metrics success by the % of healthy people who get tested?
"...the U.S. Food and Drug Administration (FDA) has finally approved an at-home rapid test for the virus. The single-use test, made by Lucira Health, can deliver results in 30 minutes."
Is this the one Musk used? The one which delivers results exactly equal to flipping a coin?
And if I test negative this morning, does that mean I'm not going to get the Wu-flu later in the day?
A handful of different assessments had been cleared via the F. D. A. For at-home and in other way numerous republican governors are ultimately, if reluctantly, wielding the strength of their reputation the coronavirus vaccine, assuming that one has been authorized by using health department
Yea, and I trust LA to be on the up and up
https://twitter.com/Peoples_Pundit/status/1328887230279069706
Only two states — California and (5.1M) New York (1M) — are responsible for the entire Popular Vote lead Joe Biden has over Donald Trump.
Los Angeles, alone at ≈ 2M, accounts for ≈ 33% of it.
https://twitter.com/Peoples_Pundit/status/1328887936373452806
Point being, those who argue it's time to abolish the Electoral College have no real argument for why we should allow two states to govern the rest of the country, the vast majority of which they do not represent.
It was their decision to use immigration to replace population.
They have a very real argument; it is the simplest way to assure the people's party of democratic socialism rules forever.
https://twitter.com/seanmdav/status/1328834157464727552
After years of FOIA litigation, the FBI finally released the 2017 FBI interview summary of Russian collusion hoaxer and Clinton campaign sub-contractor Christopher Steele.
With the exception of the first paragraph on the first page, the entire 26-page document is redacted.
The Real Presidential Election was Rigged in 2018
Every state but one that had a Democrat acting as Secretary of State was called for Biden.
https://www.frontpagemag.com/fpm/2020/11/real-presidential-election-was-rigged-2018-daniel-greenfield/
Benson was one of the beneficiaries of an initiative that started with the Secretary of State Project backed by the Democracy Alliance and George Soros. Democrats were convinced that they could have won in 2000 and 2004 if they had controlled state secretary of state posts.
Benson, a veteran of the Southern Poverty Law Center and assorted structural initiatives to benefit Democrat voter strategies nationwide, was backed by a river of cash, with her own fundraising topping $1 million, and iVote throwing in nearly another million.
iVote is a Democrat organization whose goals include mandatory voter registration, universal mail voting, and the end of any measures, like voter ID, to fight voter fraud.
Republicans were outspent 2 to 1 and Michigan got an ‘Election Week’.
While Republicans wasted money, iVote spent $6 million to rig the battleground in key states. Benson took control in Michigan, while in Arizona, a state where the Republican candidate was expected to win, Katie Hobbs, another radical leftist, won in a close election in 2018.
If Democrat States Could Ignore Trump, Republican States Can Ignore Biden
Americans have learned from Democrats that they can ignore mandates from Washington when they overstep the federal government's constitutional powers.
https://thefederalist.com/2020/11/17/if-democrat-states-could-ignore-trump-republican-states-can-ignore-biden/
The “resistance” that thrived during the Trump administration featured Democrat-run cities and states establishing so-called sanctuaries against enforcement of federal immigration law. As fears arose on the left that the Supreme Court could overturn abortion rights, blue states began passing laws that guaranteed the right to abortion up until birth. As Trump attempted to pursue a national policy to deal with the Wuhan virus epidemic, Democratic governors declared that the federal government had no right to infringe on states’ rights to deal with emergencies.
These actions and others represent a definitive assertion of states’ rights against centralized policymaking by the president. Those who might be concerned about federal infringement of rights now that Democrats control the White House and at least one branch of Congress might find a welcome irony in this fortification of a new federalism courtesy of our friends on the left.
"Ivanka Trump is the latest relative of the president to sign up to Parler, a social network popular with conservative politicians and personalities.
The businesswoman and advisor to her father, President Donald Trump, announced to her 10 million Twitter followers on Tuesday that she had joined the rival platform, which topped the U.S. iOS and Android app store download charts this month.
At the time of writing, she has not yet posted to her Parler account, which has attracted more than 200,000 followers since first being created five days ago."
----Newsweek, November 18, 2020
https://www.newsweek.com/ivanka-trump-parler-john-matze-1548277
200,000 followers in five days in a social media network that only has about 10 million people is impressive.
I wish she'd just do a lifestyle brand channel. The channel needs content that isn't about politics to go mainstream. I think the fashion and mid-tier retailers pulled the plug on her fashion brand, but she could sell all-American "Ivanka" blue jeans via Parler all day long.
You don't even need to think about the advertising. Just make it patriotic. Pro-Trump consumers are starving for some overt patriotism at this point. It's a no-brainer. Start by playing the Star-Spangled Banner in the background, and have some high-school football players standing there singing along with their hands on their hearts.
Calvin Klein and Polo Ralph Lauren used to make a big deal about being American brands--just a few years ago. Ivanka Trump could easily be the brand that has the ovaries to go full on patriotic in the face of Biden, BLM, and anti-fa. Her last name will never let her be the toast of the left and the social justice fashion world again. She might as well go all in. She doesn't need to appease the big retailers anymore anyway. That's so 2015. The malls are empty.
Taylor Swift started with Country radio in Nashville.
Do you think she has more stock than Sarah Palin did after 2008?
She had stock before Trump was elected.
She was selling hundreds of millions with of fashion and jewelry through Macy's, Neiman Marcus, and Nordstrom--before Trump was elected. They were all put under tremendous pressure to drop her lines because of cancel culture--because of her association with Donald Trump.
Now that she has millions of upset Trump voters all in one place via Parler, yeah--she should sell them some fucking blue jeans with her name on it. She has a channel. She has a lifestyle brand to sell. And most importantly, she has a channel to sell to them now. It's like shooting fish in a barrel. There isn't anybody on Parler who might not be interested in buying some all-American blue jeans to show how much they can't stand anti-American progressives.
Ivanka jeans should have an American flag incorporated into the label.
Kayne West should look at doing the same thing--with different apparel.
This is a demographic that has been horribly under-served by social media, the television media, advertising, and retail! And that is a YUGE opportunity for someone like Ivanka Trump.
https://www.townandcountrymag.com/society/politics/a22538170/ivanka-trump-fashion-brand-rise-fall-timeline/
Truth is her stuff was never all that good. Overpriced and poorly made overseas including China.
Trying to sell fashion based on politics is not going to work. Her line was sitting on the racks when she shut it down.
I'm sure you have cites for those claims; let's see them.
It's not about politics per se. It's about selling patriotism when no one else is--to the overwhelming majority of Americans who still find it immensely appealing and have done so for 200 years.
Because the social justice warriors have seized the airwaves doesn't mean their hatred of America is popular. Being patriotic has been the most reliable way to advertise to Americans since the emergence of mass media a hundred years ago.
https://www.mdgadvertising.com/marketing-insights/10-american-brands-that-get-patriotic-marketing/
Here's what Ivanka should be selling:
https://www.youtube.com/watch?v=7ZZhZBXNw9k
Barack Obama's Third Autobiography: I Read Marx and Post-Modernists So I Could Chat Up "the Ethereal Bisexual Who Wore Mostly Black" on My Dorm Floor
http://ace.mu.nu/archives/391327.php#391327
at-home tests that don't need to be mailed into a lab for processing have been banned,"
I wonder whose bright idea was that to ban something that would make life easier. Politicians and corporations
These hurdles have less to do with COVID-19 specifically, and more to do with decades-long concerns about whether people without any medical training can accurately screen themselves and interpret the results.
Thank god we have daddy government to watch over us!
I keep peeing on this goddamn stick and I still can't figure out whether or not I'm pregnant. Does anybody know if Popsicle is a good brand of home pregnancy test?
>>But at-home testing—and all its game-changing potential
GAME-CHANGING! how exactly?
Well, slobbering Joe got elected!
not yet.
"Unfortunately, this sort of counterproductive meddling has been a recurring theme throughout the pandemic (and, well, always) for the FDA. The agency has so far been holding up at-home testing over paternalistic concerns that people would not be able to properly implement the tests or interpret results, reports the Associated Press:"
And yet, people still vote for democrats.
Vote for fascists, you get fascism. Whodathunkit?
Probably not a bad idea to make the covid tests prescription only. If they put them on shelves for people to buy, you know damn well the same people who bought all the TP will buy the tests, effectively meaning the tests don't get used by the general public.
at home testing should have an app that tells the government wether you are positive or negative so they know if you can be out of the house. Why not thats why China is doing, if its good for them its better for everyone.
Some counties, states and/or regions of the US may naturally achieve herd immunity before any vaccines will be made available in limited quantity (around 20 million units per vaccine).
Percent of residents who have tested positive for covid
(note that rates have doubled in the past month in many states)
North Dakota - 8.6%
South Dakota - 7.6%
Iowa - 6.1%
Wisconsin - 5.8%
Nebraska - 5.3%
Utah - 4.9%
Idaho - 4.7%
Illinois - 4.7%
Tennessee - 4.6%
Montana - 4.6%
Mississippi - 4.5%
Alabama - 4.5%
Arkansas - 4.5%
Louisiana - 4.4%
Kansas - 4.3%
Rhode Island - 4.2%
Florida - 4.1%
Missouri - 4.1%
Georgia - 4.0%
Nevada - 4.0%
Oklahoma - 4.0%
Data posted above is from
https://www.npr.org/sections/health-shots/2020/09/01/816707182/map-tracking-the-spread-of-the-coronavirus-in-the-u-s#curves
Its about the only useful thing published by NPR.
Since July 15, the US covid mortality rate has been six times greater than Sweden's (334 vs 56 per million).
https://ourworldindata.org/coronavirus-data-explorer?zoomToSelection=true&time=2020-05-17..latest&country=USA~SWE~AUT~PRT~ITA~FRA~POL®ion=World&casesMetric=true&interval=smoothed&perCapita=true&smoothing=7&pickerMetric=total_cases_per_million&pickerSort=desc
And yet, Sweden never shut businesses or schools down (as did many states in the US, and many European countries).
Although Sweden did NOT achieve herd immunity (as was previously speculated by their Minister of Health), and cases in Sweden have been rising recently (at a similar rate as in the US), Sweden's much lower covid mortality rate during the past four months clearly shows that (to date) Sweden's response to covid were more successful than most states/nations that implemented lockdowns.
Yorn desh born, der ritt de gitt der gue,
Orn desh, dee born desh, de umn bork! bork! bork!
(or, how the CDC interprets data from Sweden)
If enough people would refuse to be tested, then there would be no basis for the COVID lockdowns.
These obstacles have less to do with COVID-19 explicitly, and more to do with long term worries about whether individuals with no clinical preparing can precisely screen themselves and decipher the outcomes.
https://dealmarkaz.pk/items/category-cameras-accessories/p-canon-700d
FDA issued an emergency use authorization (EUA) for the investigational monoclonal antibody therapy bamlanivimab for the treatment of mild-to-moderate COVID-19 in adult and pediatric patients.
https://www.gwinnettdivorce.com/