FDA Tells At-Home Diagnostics Companies To Stop Coronavirus Test Roll-Outs
The companies are complying. Customers won't get their results and are being told to destroy their test kits.
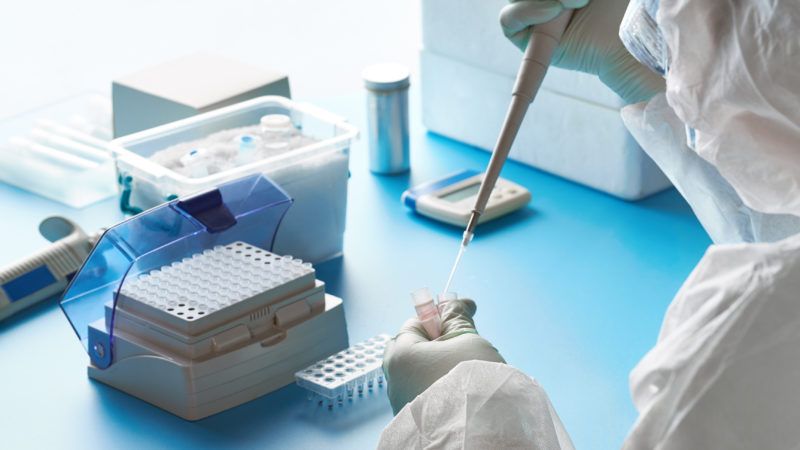
Four different diagnostics companies were planning to ship at-home tests for the coronavirus infections to their customers this week, but are instead "pausing" their roll-outs in order to avoid the displeasure of Food and Drug Administration (FDA) bureaucrats. In fact, companies are destroying samples sent back from customers rather than testing them to comply with FDA guidance.
The companies had launched their tests based on their interpretation of the latest FDA Emergency Use Authorization (EUA), which says that any lab that had been certified under the appropriate quality-control standards could start performing coronavirus tests. They were all using just such labs.
For example, to comply with the FDA's testing guidance TechCrunch reports that the California-based Carbon Health sent out a notice to its coronavirus at-home test customers stating that "we sincerely regret to inform you that you will not get a test result. If you have already shipped your kit back, the specimen will be destroyed by Curative, Inc [their lab testing partner] using standard biohazard disposal. If you have not received your kit yet, please discard it upon receipt."
Austin-based Everlywell was ramping up to handle 250,000 of its at-home coronavirus tests per week. Since the FDA's EUA does not now stop private labs from developing and deploying coronavirus tests for clinical use, the company has pivoted to making its initial supply of COVID-19 tests available to hospital and health care providers, as a way to get around the FDA's regulatory roadblock. Carbon Health is offering their coronavirus testing at its walk-in clinics.
The fact that self-administering these tests is uncomfortable may make their results less accurate, but the fact that the FDA allows their use by health care providers indicates that these tests work and are in no way fraudulent. The FDA needs to get out of the way of at-home COVID-19 testing now.
Editor's Note: As of February 29, 2024, commenting privileges on reason.com posts are limited to Reason Plus subscribers. Past commenters are grandfathered in for a temporary period. Subscribe here to preserve your ability to comment. Your Reason Plus subscription also gives you an ad-free version of reason.com, along with full access to the digital edition and archives of Reason magazine. We request that comments be civil and on-topic. We do not moderate or assume any responsibility for comments, which are owned by the readers who post them. Comments do not represent the views of reason.com or Reason Foundation. We reserve the right to delete any comment and ban commenters for any reason at any time. Comments may only be edited within 5 minutes of posting. Report abuses.
Please to post comments


Testing everyone right now is not the most important thing.
Ending the hysteria and getting back to work are the most important things.
The former would help facilitate the latter
I have made $16498 in one month by working at home. When I lost my office job 3 month ago, I was very upset and an unsuccessful try for a job hunt I was found this online job. and now I am able to earn thousands at home easily. Everybody can do this and earn money so easy.… More Read Here
It is planting season now in Georgia.
And how do we do that? Tests that show if you have or have had the virus.
Two separate tests, and we need universal access to both of them.
Testing everyone right now is not the most important thing.
I like the retards saying "Testing would calm people down." like, all of a sudden, the country runs strictly on empirical evidence.
Hitler sans holocaust is still in power but maybe if we find enough Jews who haven't been gassed to death or raped on college campuses we can convince people that the underhanded and hyperbolic media who've been doing them a disservice for several generations now aren't the axiomatic standards of accuracy and truth that we must collect mountains of unquestionable data to disprove.
Sorry. But you are wrong. The first step in defeating an invisible enemy is to make it visible. Identify. Isolate. Eradicate is the strategy.
Without sufficient testing we are flying blind. Any decision to go back to normal could, without accurate data, backfire. A national strategy that amounts to whack a mole is foolish and will harm the economy even more.
What in Odin's name could the benefit be from FDA signaling to these companies they will get in trouble for administering unapproved tests? I'm trying to imagine the FDA decision maker's reasoning here and coming up blank.
Honestly I think it comes from years of marination in a culture where you always follow procedures and rules. Literally nothing else matters: just myopically attend to the rules.
The "rules" that are the very cause of our "healthcare" crisis.
"Because fuck you, that's why." - the FDA
Except this will have a lot of scrutiny, and from people far more powerful than them. Especially if they incur the wrath of Trump and congress.
After watching Trump and Congress pitch and support the LARGEST wealth redistribution package ever to his the USA -- I've got some serious doubts..
FDA: Fraud and Death Administration. There for the protection of the pharmaceutical companies. If anybody thinks the FDA is there for the protection of the public, I have some ocean front property in Montana I'm selling cheap.
There could be a legitimate concern that the home collection of samples by those with no experience may result in more false negatives than is "acceptable". Such false negatives could cause people to feel free to go out in public and get close to others thinking they can't infect others.
It seems, however, that the FDA requiring the companies to inform clients that the test collection methods are not FDA approved and therefore the results have little value and should not be relied upon would be sufficient. Each person could then make their own decision on if they are "curious" enough to justify the cost for a test result that means little.
False negatives. That is the problem. An improperly administered test is more likely to yield a false negative, which is what we do not want.
Citation required.
CB
You could have a trial and send someone out to retest a sample of negative results to see how many were false.
Seems to me the Public Health Professionals have been a major stumbling block to getting testing going. There was the Dr. in Washington state who started testing and was told to stop. The New York Health department developed their own test and the FDA/CDC wouldn't approve it. WHO had a test but the CDC wanted to make their own and their first roll out failed. South Korea has a test as do many other places.
While Trump may have been able to override the bureaucrats he didn't. This is an example of what I think of as the real "deep state", not a conscious conspiracy but a deep institutional resistance to anything that doesn't conform to their worldview.
What; so if perfection cannot be obtained - just give up all together??!!?? As-if the FDA is perfect...
Worshiping the [WE] emperor (i.e. Federal Government) is probably the biggest curse of this nation.
While not defending the FDA's actions entirely, as someone who's worked in the at-home testing industry, I think you're both grossly overestimating the value of these results and underestimating the risk of specimen collection.
Obviously, I don't speak for the whole industry, but at least some of these test makers are mailing kits to people who are being smart and getting themselves tested *before* drinking aquarium cleaner.
Seriously, imagine specimen containers, covered in specimen, being sent through the mail. It's no joke the number of people who can't follow illustrated 4-step instructions. The 'exception' of marketing to medical professionals ensures that at least some basal level of competency is involved in the testing.
The lab may be FDA approved and CAP compliant to produce results and they may/likely even have validated at-home methods, but the idea that even a fully accredited lab can mail out sample collection materials en masse without obvious consequences is pretty short-sighted.
That's kind of like saying no one should be allowed to own a gun because some people are too stupid to use one properly. The fact that the test results are probably somewhere between "a little" and "wildly" inaccurate isn't really salient. The tests presumably hurt no one, even when inaccurate. Why not just let people buy what they want and let the market figure out which tests are fastest and most effective in the way that markets do best?
No, what the FDA is doing isn't about safety or health. It's about collecting its fee.
It’s about keeping healthcare in the USA at 30% of the entire GDP.
More cries about [WE] know better than you do..
Sell your souls to the [WE] foundation because you don't own you - [WE] own you and we know what's better for you than you do.
The benefit to the FDA, and the politicians that created it, is to control who produces and sells biological test kits. When you control who can engage in business, you've just created incentives for businessmen to give campaign cash to politicians, so they get selected as the company who can make money in that business.
It's a form of socialism; government controlling the means of production, and limiting who can produce. That's why free markets are far better - customers choose who they buy from, rather than the government choosing for you.
All the Trumpists should start forwarding this article to 1600 Penn. Ave. so that the big guy can tell the FDA to get with the program.
As many politicians have found, trying to tell the bureaucracy anything is like pissing into the wind.
They created the monster now it's time for them to destroy it. The real culprit always ends up being the Nazi Citizens among-st us that make an uproar about limiting any Hitler-arranged government.
What's next? Whining about how supplement quacks, faith healers, and koi pond additive manufacturers are being restrained from offering their medical services (curative breakthroughs) to people panicked by pandemic?
^^^Dipshit^^^
Were you unable to read the article or unable to understand the article?
"Whining about how supplement quacks, faith healers, and koi pond additive manufacturers are being restrained from offering their medical services (curative breakthroughs) to people panicked by pandemic?"
None of that applies here as the testing is done by the same licensed labs the government and medical industry are using for the CoVid19 testing . This is not some fly by night operation like you and other dishonest people are implying.
A good point that bears repeating.
Were you unable to read the article or unable to understand the article?
“Whining about how supplement quacks, faith healers, and koi pond additive manufacturers are being restrained from offering their medical services (curative breakthroughs) to people panicked by pandemic?”
None of that applies here as the testing is done by the same licensed labs the government and medical industry are using for the CoVid19 testing . This is not some fly by night operation like you and other dishonest people are implying.
Collection, however, is being done by those with (generally) no experience or training. That collection could result in far less accurate test results OR possibly even injury trying to get a swab in "too deep" in order to try to get a good test result. Such injuries would be one more unnecessary thing that the strained medical infrastructure would have to deal with.
This is optimistic.
Again, you're going to be sending samples to people who are convinced they are sick and are, for any of a number of reasons, to incapacitated to perform even simple tests fairly and accurately.
Imagine the money you could save if you just swabbed everyone in your house with the same swab and sent that kit in for testing. If one of you's got it and you're sheltering in place, what's the difference?
Those that are convinced they're sick are drinking fish tank cleaner and putting a burden on county coroners.
Arty, this is stupid, even for you.
#LibertariansForTheDeepState
Exactly what we should expect from Kirkland.
"supplement quacks, faith healers, and koi pond additive manufacturers are being restrained from offering their medical services"
I didn't know the FDA/CDC quacks were being restrained at-all!!
Four different diagnostics companies were planning to ship at-home tests for the coronavirus infections to their customers this week, but are instead "pausing" their roll-outs in order to avoid the displeasure of Food and Drug Administration (FDA) bureaucrats.
For fuck's sake...
+100000000000
Doing a proper swab is quite uncomfortable -- the swab has to go way up your nose into your throat. I expect most home users will not subject themselves to that, so the test is likely to come back as a false negative. I'm no fan of FDA overreach but this seems like a reasonable concern.
These are not the original intranasal throat swabs, these are designed to be self administered much closer to the front of nasal passages, but the FDA insists that medically trained personnel monitor the test to ensure it is done correctly. That is the current plan for drive through testing to drastically reduce the need for PPE for medical personnel. The question in my mind is why the FDA isn't simply insisting on remote monitoring video submitted to the laboratory via serialized email or included thumb drive.
The question in my mind is why the FDA isn’t simply insisting on remote monitoring video submitted to the laboratory via serialized email or included thumb drive.
This is even more invasive and complicated than what they're currently doing. It's not the FDA's job to tell them how to run their business.
If at least part of the problem is that customers can't get a swab right, how is insisting on a swab and a video going to make things simpler?
How about a face time call?
Very nearly everything the state does is either vicious or foolish, which is why the state so often appears as a cudgel wielded by clowns. ~ Aaron Ross Powell
I just bought a brand new BMW after having made $6375 this past one month and just over 12k last 4 week. This is the best and most financially rewarding job I’ve ever had. I actually started this few Weeks ago and almost immediately started to bring home minimum 74BUCKS p/h...
More Read Here
edithaiken is making twice as much.
Now Google pays me $ 22,000 to $ 32,000 a month for work from home online. Last month I received $ 27,496 on my paypal online payroll. This task is online and very easy to do part time or even full time. No special experience is required for this job. Thank you Google for giving me this extraordinary opportunity to make some money from home. This extra money has changed my life in so many ways, thank you! More information on this site ............... Read More
Looking for some added source of income? This is how you can make a decent income every month! Try it for yourself!
After being without work for 6 months, I started completing a simple online work over this website I found online,and I couldn't be happier now.
Results... After 3 months of doing this my monthly income increased by $8900 per month by working for just several hours per week...
Start by following the instructions here..............WWW.newbox3.Com
I wanna bet that by the time FDA approves a testing protocol, the COVID-19 pandemic will be over.
Just being cynical.
NO... You cannot test yourself at-home!!! Dictates the Dictators.
Now Google pays me $ 22,000 to $ 32,000 a month for work from home online. Last month I received $ 27,496 on my paypal online payroll. This task is online and very easy to do part time or even full time. No special experience is required for this job chek detail==► Read More
I am boss of my own will. Come to join under link to earn $75 per hour by watching tv with family in spare time. Earn as much as you spent time. If so please copy the link and full fill your dream...... Read More
Anything to keep the denominator as low as possible for as long as possible to conceal the true infection fatality rate.
★Makes $140 to $180 reliably online work and I got $16894 in one month electronic acting from home.I am a bit by bit understudy and work basically one to a few hours in my extra time.Everybody will finish that commitment and monline akes additional money by just open this link….. Read More
Sure. All the single-payer and out-and-out socialist healthcare systems around the world are better than the FDA. Because we have the FDA, we might as well be living in Cuba.