Of Course It's Ethical To Make 'Three-Parent' Babies
Yet Congress is keeping parents from using modern biotech to prevent disease in their offspring

Seeking to cure prospective babies of terrible diseases is clearly ethical, right? Sadly, not everyone seems to agree. Old-fashioned doctor-knows-best paternalism has all too often been replaced by bioethicist-knows-best paternalism—or worse yet, by panel-of-bioethicists-knows-best paternalism. Or at least that's the case with a promising new set of treatments called mitochondria replacement therapy (MRT).
Every cell has scores of mitochondria swimming in its cytoplasm, providing the energy that keeps them functioning. Human mitochondria have their own set of 37 genes located outside of the cellular nuclei. Because egg cells, but not sperm cells, contribute mitochondria to developing embryos, children can only inherit their mitochondria from their mothers. As with other genes, sometimes mitochondrial genes mutate and cause disease. Researchers have identified more than 250 pathogenic mitochondrial DNA mutations so far. Somewhere between 1,000 and 4,000 children are born every year in the U.S. suffering from some kind of mitochondrial disease.
Researchers hit on the idea of curing mitochondrial diseases by replacing defective mitochondria with healthy ones derived from eggs donated by other women. Back in 2001, fertility specialist Jacques Cohen and his colleagues at St. Barnabas Hospital in New Jersey transferred ooplasm containing mitochondria from healthy donor eggs to the eggs of women experiencing infertility. The experiments resulted in the births of 15 healthy babies.
The work was opposed by the usual bioethical busybodies. As the New York Times reported, "Two ethicists, Erik Parens of the Hastings Center in Garrison, N.Y., and Eric Juengst of Case Western Reserve University in Cleveland, suggested that such treatments, because they result in permanent genetic alterations that in turn will be passed on to the babies' children, might not have been approved by a federal committee that oversees experiments that involve gene transfer." The women had all been fully informed of the risks and benefits and had given their consent to the procedure, so Cohen and his team believed that they did not have to ask permission from the federal ethics gatekeepers—especially since their work was privately funded. How naive!
When the Food and Drug Administration (FDA) got wind of the new development, the agency asserted that it had jurisdiction over the treatments and promptly banned them. And that is where matters have ever since stood, as women continued to endure infertility and more babies were born suffering from mitochondrial diseases. Very ethical.
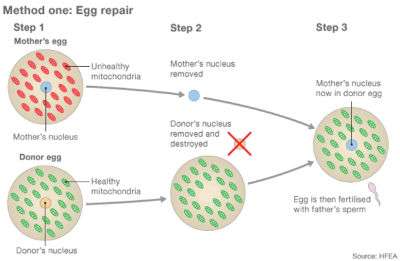
Recently, two groups of researchers have approached the FDA asking permission to try MRT as a treatment in embryos to help women have healthy babies. All of these therapies basically involve installing the nuclear genes from the eggs of women who have faulty mitochondria into enucleated eggs donated by women whose mitochondria are healthy. This would result in an embryo containing nuclear genes (99.8 percent of a cell's genes) from the mother and father and mitochondrial genes (about 0.2 percent of a cell's genes) from the egg donor.
The FDA is supposed to decide on whether to approve therapies based on their safety and efficacy. That should be sufficient: If a treatment is safe and it works, why should the agency stop physicians and patients from using it? But the FDA decided that it wanted some moral guidance, so it asked the National Academy of Sciences' Institute of Medicine (IOM) to convene a panel to advise it on the ethical issues involved.
The 12-member panel assembled by the IOM included several professional bioethicists, including Eric Juengst, who had objected 15 years earlier to Cohen's work, and Marcy Darnovsky, a bioluddite based at the Center for Genetics and Society. Their new report is out.
The good news is that the panel says that it's OK for researchers to go ahead to use MRT in cases where it is clear that women are at risk of transmitting particularly horrific forms of mitochondrial diseases. However, the panel recommended that the treatment be limited initially to creating male embryos only. This restriction means that the MRT treatment would not constitute heritable genetic modification (germline modification), since males do not pass along their mitochondria to their progeny. As researchers become more comfortable with MRT, the treatment could later be expanded to include female embryos.
The bad news is that Congress has banned MRT. After the IOM report was published, the FDA released a statement noting that Congress has forbidden the agency to spend any funds reviewing applications from researchers "in which a human embryo is intentionally created or modified to include a heritable genetic modification. As such, human subject research utilizing genetic modification of embryos for the prevention of transmission of mitochondrial disease cannot be performed in the United States in FY 2016." On the face of it, it would seem that the agency could arguably get around this congressional ban by approving MRT treatments that create only male embryos that do not pass along donated mitochondria to subsequent generations. But the agency has evidently decided not to risk angering its congressional overlords.
What about privately funded MRT treatments? Because the FDA asserted its authority over mitochondrial transfer techniques back in 2001, that privately funded research in this area cannot go forward either.
Last year, the U.K. approved mitochondrial transfer treatments. So until Congress comes to its senses, perhaps American parents who wish to avoid the risk of passing along mitochondrial diseases to their children could seek treatment in British fertility clinics.


Show Comments (55)