3-Parent Babies Born Healthy in the U.K.
The FDA blocked a similar successful treatment for mitochondrial disease a quarter of century ago.
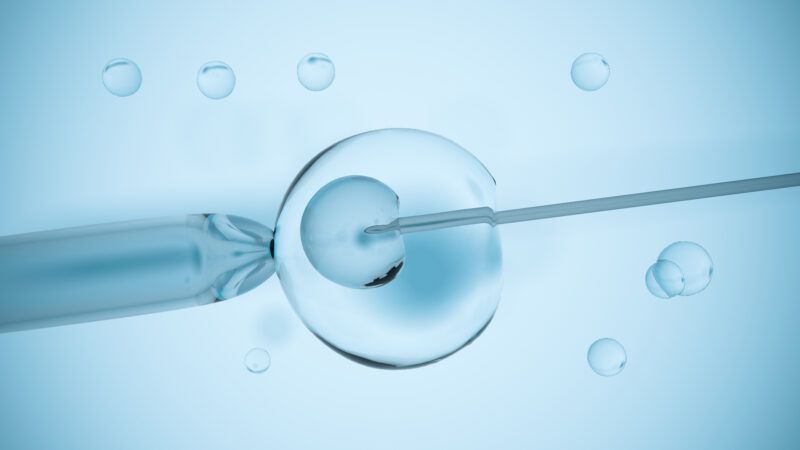
Eight healthy babies who have genes derived from three different parents have been born healthy in the United Kingdom. This happy result was reported in The New England Journal of Medicine. The treatment involved taking the nucleus from a fertilized egg that contained defective mitochondria and installing it into a donor egg with healthy mitochondria whose nucleus had been removed. Thus, the DNA comprising these babies' genes are derived from the mothers, fathers, and the egg donors. Mitochondrial DNA represents about 1 percent of total cellular DNA (about 1000 to 10,000 copies per cell). Mitochondria are the chief energy-producing organelles inside our bodies' cells. Mitochondrial disorders affect between 1 in 6,000 and 1 in 8,000 live births, making them almost as common as childhood cancer.
Many more families might have benefited over the past couple of decades from similar treatments pioneered a quarter of a century ago, except that handwringing bioethicists helped to persuade the U.S. Food and Drug Administration (FDA) to essentially ban them.
Back in 2001, fertility researcher Jacques Cohen and his colleagues at the Institute for Reproductive Medicine and Science at St. Barnabas Medical Center in New Jersey announced the births of 15 three-parent babies. In this case, the researchers injected mitochondria-containing cytoplasm taken from healthy donor eggs into those of women who were experiencing infertility.
Predictably, the usual dogmatically conservative claque of bioethicists decried the treatments. They asserted that deploying such treatments was "too important a step to be left to the consciences of individual researchers." Before being allowed to go forward, they demanded "public discussion" followed by "carefully regulated progress."
The bureaucrats at the U.S. Food and Drug Administration heeded the bioethicists' calls and issued a letter asserting the agency's jurisdiction over the treatment.
"We want to advise you that the Food and Drug Administration (FDA) has jurisdiction over human cells used in therapy involving the transfer of genetic material by means other than the union of gamete nuclei," wrote Kathryn C. Zoon, who was then the director of the FDA's Center for Biologics Evaluation and Research, in a July 6, 2001, letter. This stopped Cohen and his colleagues in their tracks.
In her 2018 Cardozo Law Review article, "How Subterranean Regulation Hinders Innovation in Assisted Reproductive Technology," William and Mary law professor Myrisha Lewis comprehensively demolishes the FDA's assertion of regulatory authority of cytoplasmic transfer and other fertility treatments.
"The FDA has been regulating cloning and advanced assisted reproductive technologies through letters for over twenty years, although it has never provided proof of jurisdiction," she concludes. "To regulate in a subterranean fashion can deter research and American patients' access to life-saving techniques." Yes, it can and it does.
How have the children born in 2001 using cytoplasmic transfer fared? Cohen and his colleagues contacted their parents in 2016 when all of the kids were now teenagers. Acknowledging the limitations of survey data, they reported that prenatal development and delivery were uneventful and that their school grades ranged from good to excellent. While one child experienced migraines and others had mild asthma, and some had minor vision and skin problems, the children overall were in good health. As Bionews summed it up: "'Three-person babies' grow up into healthy teenagers."
Had the FDA stayed out of the way, many more families would have had the opportunity to use these and similar assisted reproduction technologies to have healthy children over the past 25 years.
Editor's Note: As of February 29, 2024, commenting privileges on reason.com posts are limited to Reason Plus subscribers. Past commenters are grandfathered in for a temporary period. Subscribe here to preserve your ability to comment. Your Reason Plus subscription also gives you an ad-free version of reason.com, along with full access to the digital edition and archives of Reason magazine. We request that comments be civil and on-topic. We do not moderate or assume any responsibility for comments, which are owned by the readers who post them. Comments do not represent the views of reason.com or Reason Foundation. We reserve the right to delete any comment and ban commenters for any reason at any time. Comments may only be edited within 5 minutes of posting. Report abuses.
Please to post comments


Result of a rapefugee gang rape?
How many Cherokee mitochondria do you need in order to qualify for a scholarship? Asking for Chief Screeching White Lady.
I named my vehicle Liz Warren. White it is painted white, it says Cherokee on the back.
Is it red on the inside?
Just 1:1024 red on the inside.
Immaneedsomehelphere:
OK, so if in A Handmaid's Tale, the infertility were largely the result of diabetes and obesity, and rather than straight up impregnating the Handmaids and brutally forcing them to carry a cancerous clump of cells for 9 mos. and then birth it, they harvested their eggs while preserving the Commanders' and their Wives' genetic lineage... that wholly obviates all the rape-y, oppressive, anti-abortion paternalism and then the story is practically a fairy tale of female empowerment, right?
What about if they required all the women to fill out selective service cards in case such egg harvesting became necessary and then paid them (the ones that survived anyway) on the back end? Blissful Privileged Libertopia, no?
What if I put a chick in the story and make her lame and gay? What is it going to take to sell this political ideol... I mean story to you people?
I believe the two trains meet at 11:27 PM a mile from Akron.
LOL
Oh sure, whenever somebody *else* says "A Handmaid's Tale" everybody nods and screeches about oppressing women and the right to throw clumps of cells into blenders; but when *I* say "A Handmaid's Tale" everybody looks at me like I just recited a 10th grade algebra riddle.
Transhumanist Ronald Bailey is still salty the FDA prevented him from being born with flippers.
More seriously, how does the assertion of regulatory authority stop research? Do they declare they write the guidelines then refuse to act so everything is out of guidance?
They failed to provide guidelines so there's no guidelines for the researchers to follow nor any process for the FDA to actual review their research proposals.
More seriously, how does the assertion of regulatory authority stop research?
I don't think you understand how healthcare and thus healthcare research works. Certainly you're aware of the idea that healthcare is a human right, no?
There's a lot of "pay no attention to the man behind the curtain" going on as well. Keep in mind, this is Ron "Once we develop fake meat, we can return the ranchers' land to nature." Bailey.
I'd say see my Handmaid's Tale reference above but that's apparently too convoluted. More succinctly; the 'condition' treated here doesn't just fall out of the sky. It's the result of behavior and development dictated by the nuclear DNA. There's a case to be had that, e.g., these women should be able to buy new eggs the same way David Crosby should be able to buy a new liver and, by that same token, there are very valid ethical and biological questions about growing livers, in light of the point above about healthcare (as a human right) to support people like David Crosby.
From a libertarian or even other prospective the idea of an individual right to reproduction (considering human sexual reproduction, by any instantiation including cloning, requires at least two people) is a bit oxymoronic.
This is nothing. It's like changing an engine.
Maybe mitochondrial disease is your bodies way of saying you shouldn't reproduce. Just throwing it out there.
It's OK. It's not like we have written law declaring a woman's ability to reproduce is a natural right and the procedure takes that right and gives it to a woman who doesn't have it.
Remember when Ron MOAR TESTING Bailey was certain that there was one way our of COVID? Now he says that the important thing is that when it comes to creating lives and physically taking peoples' rights, nobody ask too many ethical questions.
Technology-averse, superstitious, highly unappealing, right-wing dead ends of evolution are the best advisors in reproductive matters.
They think the body has a way of "saying" something and derive a fully redundant, schizoid, pseudo-moral judgment from that. Just like they do with an embryo of 6 weeks.
If the Left thinks it is good to destroy their offspring when they reproduce, then who is the evolutionary dead-end?
Yeah … nah. That’s kinda dumb.
You're being sold a lie.
This isn't a treatment for mitochondrial disease. People with mitochondrial diseases aren't going to be treated with this. What this is is a way to further elucidate mtDNA diseases from nDNA diseases longitudinally. The byproduct of this is that you're going to take babies that would otherwise die of mutations or dysfunctions in one or both sets of DNA and you're going to facilitate their development to birth or further. At which point it becomes quite reasonable to ethically ask, "Is it a good idea to effectively inflict this disorder on these children for their entire lives (and any lives they may bare) rather than to not intervene."
There's a solid case to be had either way. I'm labeled as a pretty sold anti-abortion guy and even questions like "Should a woman be forced to care for a baby with Down Syndrome for life?" give me pause (I'd still question how a woman became surprise pregnant with a Down Syndrome baby but, still, it's a situation or condition to rightly consider). But Ron's dishonest "How could anyone possibly be against more fertility?" presentation is an added layer of unethical.
"Should a woman be forced to care for a baby with Down Syndrome for life?" give me pause (I'd still question how a woman became surprise pregnant with a Down Syndrome baby but, still, it's a situation or condition to rightly consider).
"Should a woman *with Down Syndrome* be forced to care for a baby with Down Syndrome for life?" Yikes!
"Should a woman *with Down Syndrome* be provided an abortion as part of free public healthcare?" HolyFuckingShit Yikes!
"Should a woman with mitochondrial disorder be able to have a baby who might require healthcare for life all on the public dime?" Uh, I know the answer *isn't* "Nah... it'll be fine."
No woman in the US is forced to care for a baby for life (or even for 18 years.) Babies can be dropped off at a police station, hospital, or other designated area.
"What this is is a way to further elucidate mtDNA diseases from nDNA diseases longitudinally."
That makes no sense. I don't think you know what those words mean