The CDC's New Pain Treatment Advice Aims To Correct the Damage Done by the 2016 Version
The proposed guidelines emphasize the need for individualized treatment and collaboration with patients.
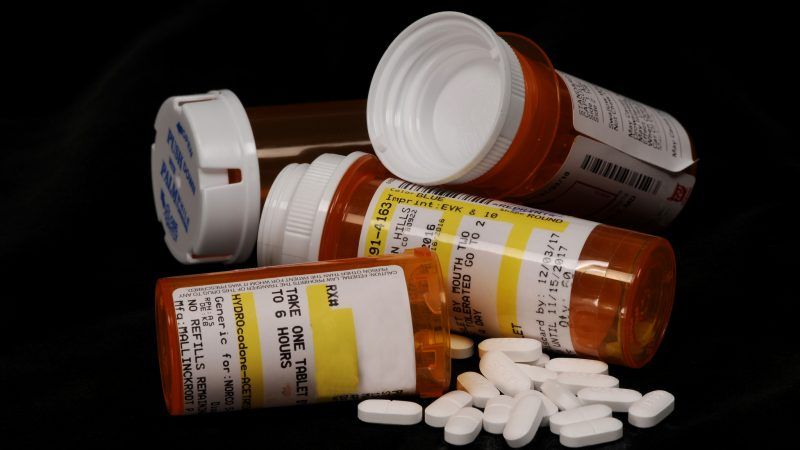
The revised and expanded pain treatment guidelines that the Centers for Disease Control and Prevention (CDC) published today mention "patient abandonment" eight times. They also include two occurrences of this admonition, in bold and italics: "Clinicians should not abandon patients."
That gives you a sense of the disastrous impact that the original version of the CDC's advice, published in 2016, had on medical care. Something clearly has gone terribly wrong when clinicians have to be reminded that they are not supposed to abandon patients. At the same time, the CDC's acknowledgment of the problem signals its willingness to address the needless suffering caused by the 2016 guidelines, which resulted in undertreatment, reckless "tapering" of pain medication, denial of care, and procrustean policies that prioritize reductions in opioid prescribing over the interests of patients.
The original guidelines, which were aimed at primary care physicians and focused on "prescribing opioids for chronic pain," included grave warnings about the dangers of exceeding 90 morphine milligram equivalents (MMEs) a day. Many physicians, pharmacists, insurers, regulators, and legislators read that threshold as a hard cap, meaning that it should never be exceeded and that chronic pain patients who were already above it should be forced to comply with this arbitrary limit.
Although the 2016 guidelines focused on chronic pain, they also touched on acute pain, because "long-term opioid use often begins with treatment of acute pain." For acute pain, the CDC said, a prescription for "three days or less will often be sufficient," while "more than seven days will rarely be needed." As a result, the CDC notes in the new guidelines, "more than half of all states have passed legislation that limits initial opioid prescriptions for acute pain to a seven day supply or less," while "many insurers, pharmacy benefit managers, and pharmacies also have enacted similar policies."
Ostensibly, the guidelines were purely advisory. But in practice, many patients found to their dismay, they were mandatory.
"Some policies that were purportedly drawn from the 2016 CDC Guideline have, in fact, been notably inconsistent with the 2016 CDC Guideline and have gone well beyond its clinical recommendations," the CDC says in the new guidelines, which are still in draft form pending public comment. "Such misapplication includes extension of the 2016 CDC Guideline to patient populations not covered in the 2016 CDC Guideline (e.g., cancer and palliative care), opioid tapers and abrupt discontinuation without collaboration with patients, rigid application of opioid dosage thresholds, application of the Guideline's recommendations for opioid use for pain to medications for opioid use disorder treatment…duration limits by insurers and by pharmacies, and patient dismissal and abandonment."
What did all this "misapplication" mean in practice? The CDC notes that the Food and Drug Administration (FDA) "has advised that risks of rapid tapering or sudden discontinuation of opioids in physically dependent patients include acute withdrawal symptoms, exacerbation of pain, serious psychological distress, and thoughts of suicide."
The CDC is referring to the "safety announcement" that the FDA issued three years ago in response to complaints from patients and pain specialists. Notably, that FDA warning mentioned not just "thoughts of suicide" but completion of the act. When patients respond to CDC-inspired medical practices by killing themselves, it might be time to admit that the agency issued its advice without sufficiently considering the potential for unintended but foreseeable consequences.
The CDC never quite admits that in the new guidelines. But it has made several notable changes aimed at discouraging "misapplication" of its advice.
"This voluntary clinical practice guideline provides recommendations and does not require mandatory compliance," the CDC says in its Federal Register announcement. "The clinical practice guideline is intended to be flexible so as to support, not supplant, clinical judgment and individualized, patient-centered decision-making."
The revised guidelines, which include 12 main recommendations, address acute as well as chronic pain and offer advice for "clinicians" generally. They explicitly do not apply to "sickle cell disease-related pain management, cancer pain treatment, palliative care, and end-of-life care." The new advice retains a bias against opioid treatment but, unlike the 2016 version, does not imply that daily doses of more than 90 MME for chronic pain are inherently suspect. The CDC emphasizes the importance of collaborating with patients, tailoring treatment, and weighing risks against benefits. It says that calculus includes not only the pros and cons of opioid treatment but also the dangers of abrupt dose reductions.
"Payers and health systems should not use this clinical practice guideline to set rigid standards related to dose or duration of opioid therapy" and "should ensure that policies based on cautionary dosage thresholds do not result in rapid tapers or abrupt discontinuation of opioids," the CDC says. "For patients already receiving higher opioid dosages, clinicians should carefully weigh benefits and risks and exercise care when reducing or continuing opioid dosage. If risks outweigh benefits of continued opioid therapy, clinicians should optimize other therapies and work closely with patients to gradually taper to lower dosages or, if warranted based on the individual clinical circumstances of the patient, to appropriately taper and discontinue opioids. Unless there are indications of a life-threatening issue, such as warning signs of impending overdose, e.g., confusion, sedation, or slurred speech, opioid therapy should not be discontinued abruptly, and clinicians should not abruptly or rapidly reduce opioid dosages."
Although these warnings are welcome (if overdue), it is not completely clear what "work[ing] closely with patients" means. "While some experts felt there should be more consideration of obtaining informed consent prior to tapering opioids," the CDC says, "others believed that informed discussion is more appropriate than informed consent when considering tapering opioids given clinicians' overriding responsibility to avoid providing treatment that harms patients."
The CDC's new recommendations do not include a warning about prescribing more than 90 MME per day for chronic pain. But the CDC does urge caution about raising doses for patients with "subacute or chronic pain" above 50 MME per day. "Many patients do not experience benefit in pain or function from increasing opioid dosages to ≥50 MME/day but are exposed to progressive increases in risk as dosage increases," it says. "Therefore, before increasing total opioid dosage to ≥50 MME/day, clinicians should pause and carefully reassess evidence of individual benefits and risks. If a decision is made to increase dosage, clinicians should use caution and increase dosage by the smallest practical amount."
The CDC adds this underlined caveat: "The recommendations related to opioid dosages are not intended to be used as an inflexible, rigid standard of care; rather, they are intended to be guideposts to help inform clinician-patient decision making. Further, these recommendations apply specifically to starting opioids or to increasing opioid dosages, and a different set of benefits and risks applies to reducing opioid dosages."
The CDC acknowledges the concern that advice tied to specific doses might lead to suboptimal care and misguided policies, as happened with its earlier emphasis on the 90 MME/day threshold. Its solution is to omit any reference to the 50 MME/day threshold from its highlighted recommendation, relegating that discussion to the "implementation considerations." Lynn Webster, a former president of the American Academy of Pain Medicine, notes that the CDC does not acknowledge the weak scientific basis for MME thresholds, which do not take into account wide variation in how patients metabolize and respond to pain medication.
The CDC clearly prefers that doctors treat pain with something other than opioids whenever that is feasible. But it acknowledges that nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen also carry risks and may not provide adequate relief. And while its discussion of nonpharmaceutical treatments may strike pain specialists as excessively optimistic, it does not insist that patients try every other conceivable option before opioids.
"Opioids should not be considered first-line or routine therapy for subacute or chronic pain," the CDC says. "This does not mean that patients should be required to sequentially 'fail' nonpharmacologic and nonopioid pharmacologic therapy or be required to use any specific therapy before proceeding to opioid therapy."
The CDC has eliminated the blanket suggestion that opioid prescriptions for acute pain generally should not last longer than a week. Instead, it says "a few days
or less are often sufficient," but "durations should be individualized based on the clinical circumstances of the specific patient." The CDC recommends starting with the lowest effective dose, avoiding long-acting formulations, instructing patients to take pain pills as needed rather than on a fixed schedule, and eschewing the practice of prescribing more pills than a patient might need "just in case" his pain lasts longer than expected.
While those recommendations may seem commonsensical, they entail tradeoffs. The CDC says prescriptions should be limited to "the expected duration of pain severe enough to require opioids." But it acknowledges that the "expected duration" is not necessarily the same as the actual duration. A patient taking hydrocodone after an injury or surgery, for example, may find that he is still in pain after his pills run out. The CDC is so keen to avoid the risk that unused pills will be diverted to nonmedical use that it is willing to let such patients suffer until they manage to get another prescription.
"To minimize unintended impact on patients with an unexpectedly prolonged duration of severe acute pain," the CDC says, "clinicians, practices, and health systems should have mechanisms in place to provide timely re-evaluation for the subset of patients who experience severe acute pain that continues longer than the expected duration to confirm or revise the initial diagnosis and to adjust management accordingly. In particular, clinicians, practices, and health systems should ensure all patients can access and afford additional evaluation and treatment, as needed, to minimize disparities across patients based on access to and affordability of care and refills."
That seems like a lot of wishful thinking. Even with "mechanisms" in place, patients will suffer from unrelieved pain while they are jumping through these hoops. There is an unacknowledged value judgment here that says the war on drugs takes precedence over patient welfare.
Likewise with the CDC's position that "opioids are not first-line therapy" for patients recovering from dental surgery. The aim here is to prevent unnecessary prescriptions that might contribute to opioid "misuse." But pursuing that goal means that many dental patients who find that Tylenol and Advil are inadequate will just have to suck it up.
The CDC's bias against opioids does not seem to be justified by the addiction risk it emphasizes. In 2015, according to the National Survey on Drug Use and Health, nearly 100 million Americans used prescription opioids, including nonmedical users as well as bona fide patients. Judging from their responses to survey questions, about 2 million of them, slightly more than 2 percent, qualified for a diagnosis of "substance use disorder"—a catchall category that subsumes what used to be known as "substance abuse" and the more severe "substance dependence"—at some point during the previous year. By comparison, data from the same survey indicate that 9 percent of past-year drinkers had an alcohol use disorder in 2015.
Nor are fatal overdoses as common as the CDC implies. A 2015 study of opioid-related deaths in North Carolina, reported in Pain Medicine, found 478 fatalities among 2.2 million residents who were prescribed opioids in 2010, an annual rate of 0.022 percent. Webster notes that the CDC dubiously blames opioid prescribing for increases in drug-related deaths between 1999 and 2010 without acknowledging that the upward trend in fatalities accelerated after the government succeeded in reducing the use of opioid pain medication. As opioid prescriptions fell, opioid-related deaths, primarily involving heroin and illicit fentanyl, rose to record levels.
Even when the CDC concedes that opioids are necessary, that message may be lost amid all its warnings about the dangers of these drugs. The CDC says "there is an important role for opioid therapy for acute pain related to severe traumatic injuries (including crush injuries and burns), invasive surgeries typically associated with moderate to severe postoperative pain, and other severe acute pain when NSAIDs and other therapies are contraindicated or likely to be ineffective." Yet The New York Times reports that the guidelines "advise against prescribing opioids, except for traumatic injuries, such as burns and auto accidents." If Times health law reporter Jan Hoffman got that impression, clinicians might as well, leading to further "misapplication" of the CDC's advice.
Overall, however, the proposed guidelines represent a substantial improvement on the advice the CDC gave in 2016. "The framing is better," says Kate Nicholson, president of the National Pain Advocacy Center. She notes "much more emphasis on the importance of treating pain, individualization, patient-centered care, disparities in care, and [the point] that the guideline should not be used as the basis for policy or a substitute for clinical judgment." She adds that "the two provisions that wreaked the most havoc—the day and dosage thresholds—have been removed from the actual recommendations."
Nicholson also has concerns. The remaining references to MME thresholds are not adequately justified, she says, and "could still be lifted by policy makers." She also suggests that expanding the guidelines to cover shorter-term pain "opens a can of worms and may confuse more than clarify." While "a lot has improved," she says, "a lot still needs to be addressed."
Bob Twillman, former executive director of the Academy of Integrative Pain Management, also welcomed many of the CDC's changes. "The wording of the recommendations themselves is much improved over the 2016 version," he told the Pain News Network. "In particular, the elimination of specific dosage numbers is welcomed because those were very easy for policymakers and payers to latch onto in setting policies."
While "it's good that they are removing those," Twillman said, "I fear that it's a bit like closing the barn door after the horse has escaped. There is a lot of work that needs to be done to modify or eliminate policies that were tied to the specific numbers in the 2016 guideline, and I'd like to see CDC play a role in that work."
Webster is less impressed by the CDC's changes. "The good thing," he says, is that "the explicit dose limits were removed and the days of opioid supply for acute pain are not specific." But "although they say that the guideline should not be an inflexible standard of care imposed on specific populations," he notes, "they do not expressly state that law enforcement and policy makers should not use them to set a standard of care or to prosecute providers."
Webster questions why the CDC felt a need to advise doctors about pain treatment in the first place. That function "should be left to professional organizations," he says. "Several experts in the field predicted the outcome of the 2016 CDC guidelines before they were issued.…The debacle with the 2016 CDC guidelines [illustrates] the reason the CDC should not be imposing their views on how pain medicine should be practiced."
Editor's Note: As of February 29, 2024, commenting privileges on reason.com posts are limited to Reason Plus subscribers. Past commenters are grandfathered in for a temporary period. Subscribe here to preserve your ability to comment. Your Reason Plus subscription also gives you an ad-free version of reason.com, along with full access to the digital edition and archives of Reason magazine. We request that comments be civil and on-topic. We do not moderate or assume any responsibility for comments, which are owned by the readers who post them. Comments do not represent the views of reason.com or Reason Foundation. We reserve the right to delete any comment and ban commenters for any reason at any time. Comments may only be edited within 5 minutes of posting. Report abuses.
Please to post comments


Will give the CDC a free pass on this. The first time in forever that they have needed to correct
misincorrect information. Carry on public health heroes!Hey, at least the CDC is surely correct about everything regarding Covid.
The
Science
Is
Settled
What part of that do you not understand?
I make 85 dollars each hour for working an online job at home. KLA I never thought I could do it but my best friend makes 10000 bucks every month working this job and she recommended me to learn more about it. The potential with this is endless.
For more detail …. http://rb.gy/u603ti
Hoping they come out with children’s propaganda such as Punky Booster kid’s show to convince youngsters to keep getting shots.
I don’t think you should encourage
buttplug that much.
So they get to like needles. No problem then...d'oh!
I am an anesthesiologist and I have been horrified at the inadequate pain relief given to post op patients.
7 days is al you get
Period.
I advise all persons to board any pain pills they have as they will definitely need them after surgery.
Why does the CDC have anything to say about this at all? Pain isn't a communicable disease.
People on pain meds should wear a mask so they don't transmit addiction to others!
If the CDC can regulate your rent, they can regulate your pain meds.
They gave people free rent, where's my free percocet?
Pain medication and even abuse of pain medication is not a disease. When the CDC was simply the Center for Disease Control, they were competent and respected. When they expanded to include ‘and Prevention’, they strayed into areas in which they are not competent. Addiction is a disease, but preventing addiction is a complex problem which might not have a solution in a free society.
I want to sort out my pain issues with my doctor. Not these assholes. They should have no say at all. In fact, we really need to start getting rid of this bullshit once and for all.
Exactly. So what if a person finds a doctor to control their addiction or ease their pain. That is what doctors are for. They idea that the government has a compelling interest here- even if these people ultimately OD themselves- is nonsense.
It is noteworthy however, that the CDC is only a part of this bullshit. The FDA has spent a long time pushing this- several years longer than 2016.
They were incompetent before they spread out, Noone noticed
Cool. Maybe this means I can get more than 6 (six) oxycodon tabs to take home the next time after major abdominal surgery. (Well, to tell the truth, it was enough, but still, it seemed rather weird)
Same here with my back and knee issues.
+
So the CDC was wrong? Again? Still?
#defundCDC
Prevent the Center for Disease Control
Well, well, well. My well's dead. No water. Hopefully it's just the controller or something up top. Don't want to have to lift the motor - that gets expensive fast.
Buuut - lifespan on these things is like 8-15 years and this one is 16ish so . . .
Check the points to make sure they are not corroded and stuck together.
The best part? By this time next year they'll have memory holed the previous guidance and pretend this has been their policy all along.
And social media will silence anyone who says otherwise.
No, social media will only silence those on social media.
Who deserve to be silenced for being stupid enough to have kept an account.
The idea that this started in 2016 is contradicted by even Reason's own reporting. Take a look at this:
https://en.wikipedia.org/wiki/Opioid_epidemic_in_the_United_States#/media/File:Timeline._Overdose_deaths_involving_opioids,_United_States.gif
Where do we see things really take off? In 2011, we see overdose deaths from "prescription" opioids start dipping. And by 2013 Synthetics have taken off.
What happened in 2011? Well in September, the FDA began working with local states to setup "the use of state prescription drug monitoring programs (PDMPs) geared to individual physicians who are high volume prescribers of opioids." (Link Below)
In 2012, this stuff continues, with research into "the prescribing habits of physicians who prescribe doses of opioids above 100 mg morphine equivalents per day. "
In 2013 the FDA voted for "rescheduling hydrocodone products from Schedule III controlled substances to Schedule II controlled substances." Once a substance is S-2, it requires much higher monitoring and paperwork for prescribers (aka Doctors)
The full link can be seen in the next post, but in a nutshell, the CDC's statement, while bad, was not the trigger. The big hockeystick started back around 2010, when the FDA went on a kick to track and harass Doctors who they felt were over-prescribing opioids.
Were doctors probably feeding an addiction? Yeah. So the fuck what? They were keeping these people much more healthy than when the FDA chased them to dealers trafficking in Fentynol.
This is all a government created problem.
Here, the FDA utterly brags about its role in criminalizing doctors and their patients, and chasing addicts into the black market for opioids. Golf clap, FDA. Golf clap.
https://www.fda.gov/drugs/information-drug-class/timeline-selected-fda-activities-and-significant-events-addressing-opioid-misuse-and-abuse
You mean it was an Obama era policy? Say it ain’t so Overt!
Yes. But the 2016 CDC guidelines on chronic pain were instrumental on getting doctors to start worrying more about prescribing opiates to their pain patients. The state of Ohio used the cdc guidelines to pass a law in 2018 that was brutal in curtailing proper pain management for patients. First the hospital system I mainly used stopped letting their internists prescribe opiates. And my pain management doc cut my opiate dosage in 1/2. No taper.
"But the 2016 CDC guidelines on chronic pain were instrumental on getting doctors to start worrying more about prescribing opiates to their pain patients. "
No that is my point. The FDA had already been issuing this guidance. By 2013 they were tracking Doctors who were "overprescribing" opioids. This has been underway for far longer.
I echo your concerns about how the medical establishment has changed. My kid had a major surgery and they gave her a single opium shot at the hospital, and made her stick to ibuprofen at home. It still boils my blood to think about how she spent 2 days in near constant tears from the pain. That could have been avoided, but the Fed, State and localities have these healthcare systems terrified to prescribe opiates.
I also have family who I am reasonably certain are addicted to opioids. They are older, and hard laborers. They spend all day hauling shit out of trucks and constructing buildings. At the end of the day, it all hurts. And 10 years ago, they worked with their doctor to get pain relief. Today, they probably go to some shithead on the corner. And I am in constant fear that one day, police will get their normal "pain pills guy" and they will go to someone new who will serve them up a drano-fentynol cocktail.
Last surgery I had, year before last, as I was being wheeled to the car after waking up, I asked the nice nurse lady what she was giving me for drugs. She said fentanyl. It was kinda nice. Like floating. But after that all they did was suggest alternating Tylenol and Advil. Shit hurt. A lot. For weeks.
1st, you need to realize no one can BECOME an addict. You either are or you aren't. It's a mutation on the G188A gene, the mu3 (opiate) receptor. If you're prescribed medication and haven't had a problem in the past, you won't this time. Harvard and the BMJ followed 500 THOUSAND post op patients and discovered the rate of misuse was... .06 percent. Meanwhile we have people killing themselves left and right because of pain. Untreated or under treated. Knock off the propaganda, and dig in. Just because you know someone who's an addict doesn't make it good policy. In fact, it's inhumane and no one gives a shit about pain. Take ibuprofen that kills your GI and kidneys. But don't take too much, they warn. Asshats.
Today is one of the greatest days in recent American history: it’s Mask Liberation Day all over the country, as the evil, despicable mask nazis have surrendered to the forces of freedom and liberty.
Unfortunately, fuhrer Anthony Fauci probably won’t face the modern day Nuremberg trial he so richly deserves, but we can all celebrate our liberation nonetheless.
Today is one of the greatest days in recent American history: it’s Mask Liberation Day all over the country, as the evil, despicable mask nazis have surrendered to the forces of freedom and liberty.
I'll let you know when my state lifts its mask mandate. And covid passport mandate... and...
In his defense, he was against the masks before he waa for the masks.
I won’t celebrate till my kids don’t have to wear one at school.
We stopped worrying about masks over a year ago so I don't know what you're talking about
He might be talking about Commifornia, or Taxachusetts, or the People’s Republic of Maryland.
On one level, you can say, “Fuck these people. They deserve it.” On another level, you could recognize that 30%-45% of the people in these states want no part of this bullshit and are groaning under the yoke of their progressive overlords.
In Free America, we are celebrating the second anniversary of not having mandates.
The science has re-settled.
IIRC: Shortly after the CDC issued the 2016 guidance at issue, the DEA publicly announced that they considered the new guidance a hard rule and that they would prosecute any doctors who did not comply.
The DEA was persecuting chronic pain patients and the doctors who treated them long before the CDC issued the guidance at issue.
But at least it solved the opioid crisis.
As she explained, Diane you twit, the "circumstances have changed" - the Omicron surge is dissipating as predicted and especially in the northern blue states where it 1st hit - and "the science has changed" as she also explains because - as hoped for - the vaccine boosters proved effective against Omicron.
This is not the 1st time the CDC and Biden recommended a lifting of mask requirements due to improving circumstances, as they did that in April of 2021. Of course this fact is counter to the MAGA/GOP fairy tale that Biden and the CDC and others tasked with dealing with the pandemic just want the power over our lives, as if they masturbate to the rush they get every night imagining us all suffering under their edict. Get another fairy tale!
Are you stupid or just a propagandist?
Boosters had little if anything to do with it. OMG variant is just less deadly (while being more transmissible).
That's false Bruce. Those with boosters have been a considerably lower number of new cases and hospitalizations since Omicron became dominant then the unvaccinated of course, but also of those with one or 2 vaccines.
Nothing has changed, Joe, except their internal polling numbers.
"Opioids should not be considered first-line ... therapy ...," the CDC says. "This does not mean that patients should be required to sequentially 'fail' ... nonopioid pharmacologic therapy ... before proceeding to opioid therapy."
Is that so?
Sullum, good article. It would be good to see a follow up on the genesis of all these regulations. Other commenters mentioned events from the 2010's. The amount of suffering and misery (and death) caused by government imposed regulations wrt opioid pain-killers is substantial.
The author minimizes the seriousness of the opioid addiction problem in America, which I have 1st hand knowledge of. An excellent lead carpenter/supervisor for me - conscientious, smart, hard working, and skillful - messed up his knee in an off work incident and was prescribed oxycontin or some similar pain relief medication. Within 6 months - he had access to my accounts at suppliers across town as part of his work - he was charging tools and pawning them to get money to buy on the street. At the same time a client of mine's son went through a similar experience with her son. This was 10 years ago, and I don't know if either of them has ever come out of it. It is serious stuff and an over powering addiction which ruins lives.
The reason the CDC gets involved in this kind of issue is that doctors are busy with patients and don't have time to research or find unbiased information on the latest best practices for the myriad of problems they must address with patients every day, and they can get plenty of loaded information from drug reps who are always looking to sell their wares. Clearly there was a national level of ignorance among doctors on the seriousness of seemingly innocent prescriptions for pain relief that led to much abuse and the ruining of lives. Of course we want clear eyed and intelligent research into best practices and an information clearing house for docs not run by guys promising weekend vacations in Bermuda if you use their stuff.
"Within 6 months - he had access to my accounts at suppliers across town as part of his work - he was charging tools and pawning them to get money to buy on the street. At the same time a client of mine's son went through a similar experience with her son."
It sounds like you are poorly managing your account security. That an asshole decided to rob you is not reason to chase millions of people into the black market, when they are managing to use pain pills without robbing their employers (or other law breaking). Since the FDA and CDC started turning the screws on Doctors, opioid overdose deaths have skyrocketed. A real hockey stick.
The point of "Reason" is to avoid causing more harm than you are hoping to prevent. Instead, the CDC and apologists like you are emoting and frantically insisting that we must "do something". This has real world consequences that you cannot ignore. It is not enough that people were ruining their lives before. They are dying on black market drugs now, and that is largely due to the CDC and FDA making legal and safe drugs illegal.
As a terminal cancer patient addiction isn't a huge concern to me. I haven't upped my precription levels in a couple years. No they don't work as well as they used to but part of that is also the progression of the disease. They have bought me a few extra years of normal living of which I am grateful. At some point something else will need to be done. And yes running out, or delays in getting refills gives you some miserable days. I totally get why some people have an awful time. They are definately something that needs a long slow taper. But it's a risk/reward case. I'd be fine if something else worked as well. Doc and I tried some other meds and they didn't come close. So until these CDC idiots get to experiance what I go through they should keep their noses out of it. I'll be gone soon enough, Being able to function in the mean time is my main concern. We can all see how well the CDC is doing with Covid, please stay out of my loop until you figure that out.
Point is, none of the interventions or policies have solved or improved the addiction problem. And people who legitimately need pain medication to function are often being denied proper care.
Zeb, you point out a valid problem, but it is partly in a reasonable reaction to the other problem of those who legitimately needed pain relief becoming addicts due to doctors over prescribing opioids. These are both legitimate problems.
Yes, but the "solution" you appear to favor has been shown to make both problems worse.
While the problem(s) are not solved and in many ways are worse, there are many factors at work and laying it at the feet of the CDC more ideological than fact based.
Whose diktat shut the oxy nozzle again?
You apparently have never been on the other side of this, with a loved one with chronic pain (in my loved one’s case - 5 replaced disks and over a dozen back and neck surgeries, from an auto accident). Aspirin, and equivalents, cause bleeding in the stomach, while Tylenol use had their kidneys shutting down. The pain causes blood pressure to go through the roof (and a history of brain bleeds in the family).
These public health bureaucrats not only get between patients and their doctors, they make the job of even the best pain doctors almost impossible. If you have a hard-to-find pain med (in our case, one that is less addicting) you may be OTL if your normal pharmacy has switched suppliers. You no longer get paper scripts that you can take to another pharmacy, but instead must use an electronic one, that cannot be transferred to another pharmacy, even in the same chain. The doc can cancel it, and issue another one to another pharmacy, if they haven’t made this mistake before this month. Try checking around for availability - for most pharmacies, you need the script to check their inventory, or even their suppliers. Catch 22. The public health bureaucrats don’t care. It isn’t their chronic pain.
Bruce, you are correct that I have not personally experienced that side of the problem and I fully acknowledge the reality of it and you have my full sympathy and wishes for relief. My understanding though is that it is the DEA, not the CDC which has the power to regulate these drugs. The CDC studies and prepares recommendations, and as technocrats and "bureaucrats" are not motivated by selling product but by pursuing their chosen area of expertise. I agree with a need for that expertise for reasons I gave above. Doctors do not have the time to do this research nor can they rely on companies selling product for guidance on it's effectiveness and use. It seems that much of your complaint has more to do with modern electronic pharmacy practice than anything else.
"The reason the CDC gets involved in this kind of issue is that doctors are busy with patients and don't have time to research or find unbiased information..."
LOL
'Unlike medical doctors, with notable exceptions, government researchers are an incredibly diverse...'
I believe that you are greatly oversimplifying the issue of addiction. Despite what has been seriously (and likely intentionally) misrepresented by our corrupt media.
There are numerous other types of addictions that cause significantly more societal damage than opioids.
As a matter of fact, it has been well established by none other than the CDC that "addiction" rarely occurs in patients experiencing pain. More specifically, only +/- 2% of pain patients ever experience any type of opioid addiction.
It's part of the false narrative the corrupt media has been pushing for decades.
Overt, clearly you do not employ anyone or if you do, no one worthy of trust to deal with the details of your business that would limit your abilities to oversee the big picture if you had to do it instead. The truth and point of my post is that the supervisor was not an "asshole" until he became an opioid addict, again a simple and oft repeated event across America which you lack the knowledge to understand.
The data on this problem is affected by multiple and complex factors but one fact remains, which is the over prescribing without limits and oversight of opioids by doctors in the oughts. Get back to us when you read up more and understand the problem. I lived through one tragic episode in it. Another fact is the pushing of drugs by profit motivated reps and companies on docs too busy treating patients to research the latest. That's one of the things the CDC does.
This horse is well out of the barn. And they have only partially closed the door. Law enforcement has decided they know how to control opioids, so they have used the CDC recommendations to arrest physicians. Do that once, people notice. Do it twice (they have) and any doctor in his right mind would either pay absolute attention to the strictest levels set, or stop prescribing pain meds altogether (like me). You would be crazy to exceed those initial levels, even if the CDC has partially walked them back.
Those initial recommendations will stick forever. If the FBI decides they want to get you for prescribing opioids, they will use those recommendations to prosecute you. It doesn't matter if you eventually win in court. The mere accusation will destroy your life.
The only cure is for the CDC to issue a declaration that all of its preceding declarations were false. That won't happen, so patients will suffer.
I understand that in other countries it's not this bad. Most other things about medicine in those countries might be worse, but not narcotics.
Too bad in the USA pain treatment gets divorced from being just one of many aspects of care, into something you have to go out of your way to get. Can you think of any other symptom that gets such special "treatment"?
How many people have suffered and died in the last four years because of this insane policy? I’m glad someone finally figured it out. Now let’s see if they are as adamant to correct their misinformation and make sure people are treated like human beings with serious medical conditions and not drug addicts,
CDC should have this reviewed by a compliance lawyer, because at least half of the problems with the 2016 guidance were people and organizations looking at it as a compliance requirement and interpreting it in that way.
What will a compliance lawyer tell his client they have to do? And if that is not what is intended, change the document to make it more clear.
All four wisdom teeth out in one session was when I found out no oxy. Very, very unhappy couple days post-op. The dental advisement was absolute shit.
I personally think opiods should be a last resort and I loathe the feeling of them, but they have their place when you need to be comatose for a length of time and that determination should be by the overseeing physician, subject to appropriate periodic review to keep them honest and put the Sacklers in jail.
Well great, now, as far as the DEA and therefore doctors are concerned, they've gone from imposing a 90mme/day cap to a 50mme/day cap.
"Many patients do not experience benefit in pain or function from increasing opioid dosages to ≥50 MME/day but are exposed to progressive increases in risk as dosage increases,"
is outrageously misleading as "many" means small very small percentage. It's disgustingly unethical and dishonest to claim that tolerance essentially doesn't exist past a dose so small there's single pills that exceed it, and instant release pills where 2 exceed it. Risks go *down*, not up, as tolerance to respiratory depression outpaces tolerance to pain relief, and the 'risks' stay flat except for considerations like "how bad will their withdrawal be when we yank it away" and "how much will their meds be worth if they sell them". They're no doubt alluding to the oft-repeated "opioid induced hyperalgesia" issue that effects "many" patients in the same way other rare disorders exist, but shouldn't dictate treatment courses for the vast majority who won't have that happen.
Because despite the disclaimer, the only reason to include a medically arbitrary dosage limit above what an opioid-naive patient would take is to intend it as a cap, and while their little disclaimer may make some prescribers willing to go to 90, you bet high dose therapy is still out of the question.
And what good is advice about abrupt tapering after they've already kicked everyone off high dose therapy and their 50mme cap will make sure nobody besides the terminally ill gets it?
It's clear the CDC has changed very little and still intends to make life hell for chronic pain patients. Chronic pain shouldn't have even been touched in 2016. The inappropriate prescribing revolved around acute pain, short term pain, and criminal operations.
The CDC, FDA and all the other acronym government agencies are completely ethically and morally corrupt. You should read what the Harvard center for ethics says about guv agencies like this.
As a chronic pain patient of 40 years. I started having migraines at age 22 I’m 62 now. I’ve had my first cervical spine fusion in 1996, and two more followed. And failed to stop the pain. I later went to a spine team facility and made a surgeon cry. He showed me from another surgery leaving out that patients name how my surgeries should have been done. I’ve been SSA Disabled since 1997. My life of working for Doctors since I was 18 done. Fighting Depression over what was lost. My Faith keeps me strong. I had a 4th surgery an implant that was successfully and necessary. I have blown discs L2-S-1 low back. And Fibromyalgia. Arthritis entire Spine. I’m never out of pain. To go to pain management you sign a minimum 5 page contract to do as they say. Take the meds as prescribed get injections to my spine every 2 to 6 months under sedation and pee test monthly like a criminal for drugs I’ve never heard of or would never ever consider taking. Then after 6 six years of driving 1 one hour one way, a 2 hour round trip to said pain management doctor, I was dumped by her because she claimed she could no longer help me. Yes, the CDC has screwed over patients like me. I’m told to take extra strength Tylenol or Ibuprofen. Do you have any clue what that is doing to my Liver much less the FACT I’m still in Daily Ridiculous Chronic Pain. Something has got to get better for people. This is 2022. Not the dark ages! You’re dealing with peoples quality of life. My daughter in law invited me to go to Church with her and my granddaughter yesterday. She was willing to pick me up. Go out of her way for me because she is a lovely person. I was in too much pain to go. That is flat out sad.
This is not going to help. It is about 200 pages of smooze until they drill down to what they really intend to do. 50MME will be the new 90MME. They are telling people with migraines use chamomile oil. They know nsaids and tyelenol are bad but take them anyway. Use gabapentin & lyrica (toxic but do it anyway) They will use this to implement the National Pain Strategy and you will have to accept the label of addict just to get Buprenorphine because that or low dose naltrexone or suboxone is all anyone will be able to get if at all