A 'Right to Try' Bill Is Finally Heading to Trump's Desk
It's still not clear whether pharmaceutical companies will work with patients outside the FDA's supervision.
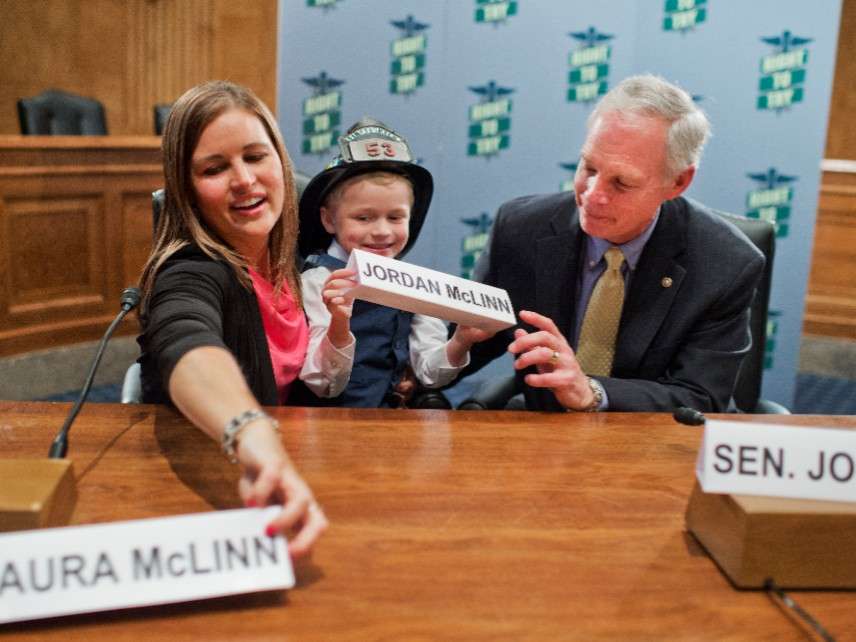
After months of debate, the House of Representatives on Tuesday passed a bill that will allow patients with life-threatening diseases to use experimental treatments without the approval of the Food and Drug Administration. The Trickett Wendler, Frank Mongiello, Jordan McLinn, and Matthew Bellina Right to Try Act of 2017, which the Senate approved in August 2017, now heads to President Trump, who has promised to sign it.
To be covered by the bill, treatments have to complete Phase 1 of the clinical trial process, which is the most basic safety assessment. The bill does not compel doctors to participate in Right to Try treatment protocols. Nor does it require pharmaceutical companies to provide experimental treatments to patients who request them.
An earlier House version of the bill was restricted to patients who had reached "a stage of a disease or condition in which there is reasonable likelihood that death will occur within a matter of months," or who were suffering from a disease "that would result in significant irreversible morbidity that is likely to lead to severely premature death." The version approved this week, by contrast, covers anyone with a "life-threatening disease."
The bill provides some legal protection for both doctors and drug companies in the event that an experimental treatment hastens a patient's death or causes some other undesirable side effect. The bill also requires drug companies to report any adverse effects to the FDA. Right to Try proponents say reporting such outcomes will not affect the approval process for the drug unless an adverse reaction reveals a fundamental danger.
Whether passage of this bill is a good news for patients depends on whom you ask. Medscape has rounded up reactions from various corners of the health care world. The conventional wisdom says no one should consume anything that has not completed all three phases of FDA clinical trials and been approved by the agency. A sample reaction:
The legislation "opens the gate to a dangerous, uncharted pathway for accessing experimental medications that have not been shown to be safe or effective," said Michael A. Carome, MD, director of the Health Research Group at Public Citizen, in a statement. "The bill passed today will expose vulnerable patients to risks of serious harm, including dying earlier and more painfully than they otherwise would have, without appropriate safeguards."
Carome and other opponents of the bill argue that the FDA's "expanded access" program (a.k.a. "compassionate use") does everything Right to Try would while providing an additional layer of patient protection. Other critics have argued that Right to Try will divert patients from clinical trials. These talking points are at odds with each other: If patients don't need Right to Try because they can already obtain unapproved treatments through expanded access, but expanded access is not draining the pool of clinical trial participants, why would Right to Try?
The libertarian Goldwater Institute, a leader in the contemporary Right to Try movement, is very pleased:
"Today's vote is a win for patients. Millions of Americans who have been told they are out of options and it's time to get their affairs in order, are closer to having the opportunity for one last treatment, without having to get permission from the federal government first," said Victor Riches, president & CEO of the Goldwater Institute. "Members of Congress came together to put patients first and we're grateful for their support for this bipartisan, grassroots movement powered by real patients in all 50 states."
The free market Heartland Institute also supports the bill. "Now, at long last, American patients and their families can have hope that a life-saving drug will no longer be denied to them because of bureaucratic barriers," Heartland President Tim Huelskamp said in a statement emailed to reporters. "They will no longer be barred from trying to save their lives."
I think Robert Graboyes at the Mercatus Center has the most concise summary, contained in an email from Mercatus, of what's at stake:
Effectively, the law grants patients, doctors, and drug manufacturers greater decision-making authority and greater capacity to assume risks. The practical importance of the law remains to be seen. Opponents of the new law argue that the FDA's compassionate use waivers already get investigational drugs into patients' hands early on; supporters argue that the administrative burdens of the waiver program discourage its use. Now, we'll get a better idea of who is correct.
Results will be interesting. The FDA has always had a seen-and-unseen problem in its incentives. Patients who suffer adverse outcomes from using experimental drugs are relatively easy to identify, while those who suffer for lack of experimental drugs are largely unidentified and unseen. In more concrete terms, it's easier to point a TV camera at the first group than it is at the second group. That asymmetry could still frustrate the intent of the new law. Time and experience will tell.
Earlier this year, I dug a little deeper into why a federal Right to Try bill might not move the needle much. The short version is that incentives are aligned for pretty much everyone except the drug companies. Some patients might get to try treatments that could extend their lives, and their doctors would get to bypass insurance companies (because no insurer is going to touch a Right to Try treatment) and forgo the FDA's paperwork.
But I'm not sure what's in it for drug companies. Right to Try data won't be randomly controlled, which means they won't help make the case for FDA approval. An adverse outcome, meanwhile, could hurt a drug's chances of approval even if its role in that outcome is hard to measure in an uncontrolled treatment setting. As Graboyes says, the only thing we can do now is wait for the market to react and hope for the best.
Editor's Note: As of February 29, 2024, commenting privileges on reason.com posts are limited to Reason Plus subscribers. Past commenters are grandfathered in for a temporary period. Subscribe here to preserve your ability to comment. Your Reason Plus subscription also gives you an ad-free version of reason.com, along with full access to the digital edition and archives of Reason magazine. We request that comments be civil and on-topic. We do not moderate or assume any responsibility for comments, which are owned by the readers who post them. Comments do not represent the views of reason.com or Reason Foundation. We reserve the right to delete any comment and ban commenters for any reason at any time. Comments may only be edited within 5 minutes of posting. Report abuses.
Please to post comments


Fucking right-wing, racist, anti-science, Jesus-loving, do-nothing, totalitarian thugs!
Seriously. Thanks a lot Drumpf. His "right to try bill" is just a hidden way for him to deliver xyclon b to disadvantaged communities. Literally Hitler's gonna literally Hitler.
#RESIST
Actually, I expect this to turn out as: How could the GOP allow evil drug corporations to do this to poor inner city minorities?
He might sign it just to piss off NPR.
clearly his principle motivating factor...anything that pisses off NPR is +1 in my book
Start earning $90/hourly for working online from your home for few hours each day... Get regular payment on a weekly basis... All you need is a computer, internet connection and a litte free time...
Read more here,.... http://www.onlinereviewtech.com
This is where the "xyclon b" should be delivered.
Start earning $90/hourly for working online from your home for few hours each day... Get regular payment on a weekly basis... All you need is a computer, internet connection and a litte free time...
Read more here,.... http://www.onlinereviewtech.com
fg
>>Right to Try bill might not move the needle much
gotta start somewhere...not allowed to burn down FDA, so this...remain optimistic.
At least it's a start. If I have a recurrence and my only option is a trial drug that I don't qualify for, I'd prefer the option that might save or prolong my life, even if the trial involves long odds.
Who is going to pay for these drugs and why on earth would a legit company jeopardize a clinical trial by selling in an uncontrolled environment that is going to generate adverse events?
Every one of these patients is going to be reported as an SAE.
There are ways. The trial criteria often are about strictly controlling the variables. If someone gets the drug outside the trial, it's easy enough to paper that up to avoid liability and to disclaim (if it gets out) that this indicts the drug itself.
This already goes on some of the time, anyway, with compassionate use exceptions. Also, some of this is about off-label uses of existing prescription drugs.
That isn't how it works.
These will all get reported as serious adverse events to the FDA.
There is a reason that Pharma companies develop "tool compounds" that they use in late phase animal studies once clinical trials have begun. If you kill animals with the candidate drug, you have to report it.
Likewise, we don't provide unapproved drugs to independent investigators prior to approval. We do not want to generate a bunch of adverse events that we can't explain in the months leading up to fda review.
Imagine the scenario. The fda advisory committee meets tomorrow. CNN reports today that 13 people have died while taking your new medication.
The committee asks "wtf brosef?" Your only response is "fuck if I know."
That is not a day that ends well for the Pharma company.
I can only imagine this working in a scenario where a drug is approved in Europe and for whatever reason the Pharma has abandoned plans for US approval.
FDA would have to allow ordering the drug from Europe. The Pharma co wouldn't be involved.
But people do die in trials, especially Phase I.
people die...period, just a question of when and maybe how...
No they don't. Phase I is often healthy volunteers, and they are started on sub-therapeautic doses that are escalated slowly to monitor for safety and pharmacokinetics.
Thanks, Bubba. You clearly work in/understand the industry. Every one of these cases would be picked up by pharmacovigilance, and they'd have to write up a separate report for each and include them in their annual PV reports. Without vetting the investigator/physicians, you're just going to end up with a lot of questionable data that looks bad.
A lot of the process of bringing a drug to market has too much politics involved (especially regarding the length of time), so if a company is relatively assured of the benefits of their drug then they might see some benefit in currying goodwill with the bureaucrats and healthcare providers.
That's not really the case. Especially for disorders that currently have no cure, the bar is set much lower for initial approval. The amount of scientific evidence that is necessary for approval is an ongoing conversation with the FDA that changes over time based on how good the data look.
Someone who wanted to offer a putatively therapeutic or diagnostic product at no charge in either intrastate or interstate commerce, when that product wasn't protected by someone else's patent & wasn't the subject of an INDA, was already legally allowed to do so. Someone could also legally charge for the service of custom-mfg. a drug or device for a buyer's club of pts. There were a lot of things people were legally allowed to do, but hardly anybody wanted to do them.
The trouble is pts. who wanted to have their cake & eat it too. They wanted non-commercial access to a drug or device that was already the subject of someone's publicized testing for FDA submission, & was already mfd. to those stds. But why would someone conducting such testing want to release the product in such a manner?
This is the trouble w a lot of proposed & enacted food & drug reforms. You try changing 1 aspect, you don't realize that the other aspects in effect occupy the same space.
Because before 1962 the FFDCA didn't explicitly require a demo of efficacy for new drugs, & because new drug introduction slowed down a lot around then, some people think that if the explicit efficacy requirement were repealed, NDAs would become a lot easier. Guess again. In determining the safety of a drug, FDA was already taking efficacy into acc't in the risk-benefit analysis, & would continue to do so in the absence of such an explicit statutory requirement. The increase in cost & difficulty of NDAs was not from that, but just gen'l regulatory thinking.
There were a lot of things people were legally allowed to do, but hardly anybody wanted to do them.
So then a law not forbidding them from not doing that shouldn't be a problem then, right?
You try changing 1 aspect, you don't realize that the other aspects in effect occupy the same space.
Right. The way we have *both* an FDA *and* a DEA.
Useless and obvious redundancy is systemic, so may as well just accept it, right? Socialized medicine or GTFO!
Shouldn't be a problem, but shouldn't be a sol'n either.
What the fuck is a "sol'n" Hihn? Do you do that to try to disguise your writing?
It didn't work.
A "sol'n" for whom, exactly?
Unfortunately the easiest achievable reform these days that'd be likely to have some effect would be the raised eyebrow of the admin. In other words, lean on the scale to favor findings of acceptability of products, while claiming to be administering the statute the same as always. & keep Trump & like-minded prezs in office. Stack the advisory boards w secret laisez-faire types.
You might be able statutorily to get some marginal improvement via reciprocity w other countries, amending the FFDCA to allow import'n & interstate commerce in products if they're allowed in other countries. Not sure the voters will go for that once they hear the propaganda from the respective interests.
Not going to happen. The charter of the FDA is safety.
They have a statue of the person who blocked thalidomide's approval.
All drugs are toxic so the question is always risk/benefit. And they won't admit it, but cost gets thrown in there too.
No wonder. That was over 60 years ago. What have they done since? Do you see millions of people with flippers for arms walking around in Europe? I didn't when I went to Italy a couple years ago. Thalidomide was legal there.
yes, yes I do....fucking flippers everywhere...I blame dolphins...
"import'n "
this a stupid attempt too Hihn, give it up it isn't work'n
wa?
Why, does Hihn not abbr.?
There was talk a few decades ago, mostly w.r.t. RU486, of a state-by-state movement. State pharm laws are written in a way to require the st. pharm bd. to license drugs except where they may legally be sold in interstate commerce. Nobody wanted to go get a license from each st., when they could get one from FDA. But that expensive escape valve is still there in case FDA becomes hard-ass. But there's no evidence that that's the problem these days.
Fuck off Hihn.
For the love of God, is it THAT hard to write out "state"?
ya!
This is a step in the right direction with positive results for many people.
thought SAE was shorthand for Self Addressed Envelope...hmmmm
When people are dying and desperate, they will do anything. People will try not only unproven treatments with some science to back-up that it might work vs complete snake oil that costs a lot of money, but provides no relief or cure.
One of my relatives had cancer. After surgery, the doctor really wanted her to join a chemotherapy protocol. When they asked about the likely benefits, the benefits were to advance science. For my relative it meant sickness, long drives to the treatment and a waste of the time she had left. They declined the protocol.
If the treatment is untested (or still being tested) there should be no charge. Doctors and researchers get useful data, but no financial incentive to push unproven treatments on the dying. If there are serious side affects, that should be made clear as they might be giving up the few good days they have left for a lottery ticket.
The legislation "opens the gate to a dangerous, uncharted pathway for accessing experimental medications that have not been shown to be safe or effective," said Michael A. Carome, MD, director of the Health Research Group at Public Citizen. I think "not been shown to be safe or effective" doesn't really matter does it? I've been in favor of this ever since the government forced Steve McQueen to die without trying the holistic treatment he wanted because it probably wouldn't prove helpful. I agree, it probably wouldn't have but how was that the business of anyone but Steve McQueen?
This sounds like advertising to get people into clinical trials while being able to charge them instead of saying it's unethical to pay people well to be in trials. Pharma has a hard time getting people into trials with the promise of free drugs. So tell the people you'll allow them to take the experimental drugs insurance won't pay for and manufacturers don't have to give. Instead of "we need you to join our trial" they change the supply and demand to "we want your phase II, III, IV drug early, we'll give you anything".