The FDA Finally Approves a Real At-Home COVID-19 Test
Way late, but better than never.
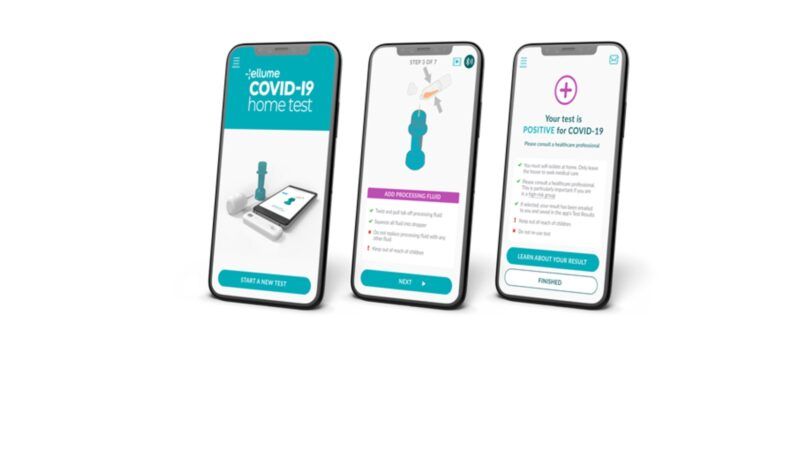
Good news! An at-home test for the coronavirus is going to be available to consumers. The Food and Drug Administration (FDA) just approved the Ellume at-home diagnostic antigen test for COVID-19. Using a smart phone app, the test detects in about 15 to 20 minutes coronavirus proteins obtained from a nasal swab. The tests will be sold over-the-counter to consumers and cost about $30 per test. The at-home test correctly identified 96 percent of positive samples and 100 percent of negative samples in individuals with symptoms. In people without symptoms, the test correctly identified 91 percent of positive samples and 96 percent of negative samples.
The bad news is that at-home diagnostic testing took way too long to get developed and then approved by the FDA.
Consider that the COVID-19 vaccines that will blunt the course of the coronavirus pandemic toward the beginning of next year were developed as part of the federal government's Operation Warp Speed. Had a parallel strategy of mass testing based on the development of cheap at-home COVID-19 tests been similarly pursued, the pandemic's toll of deaths and hospitalizations would have been much lower. Why?
In a thought experiment, Washington Post columnist Megan McCardle explains how testing could have spared millions of Americans from the disease. Start with 100,000 pre-symptomatic and asymptomatic infected people who will, on average, infect 3 other people. If that 100,000 had taken advantage of a COVID-19 test that detects only 90 percent of infections, that would still catch 90,000 cases. Being responsible individuals who do not want to risk infecting other non-consenting people, the 90,000 with detected infections would then voluntarily isolate themselves. So instead of 300,000 new infections, the 10,000 undetected folks transmit the virus to only 30,000 other people. In the next round of testing, the 3,000 undetected cases transmit to just 9,000 other people. And so forth until the epidemic is essentially crushed.
Ellume aims to produce more than three million tests in January 2021. That's not nearly enough, but it's a start.
Show Comments (56)