'Normality' Draws Closer as FDA Panel Recommends Approval of Johnson & Johnson COVID-19 Vaccine
Adding a third vaccine could get America back to something resembling normal by this spring.
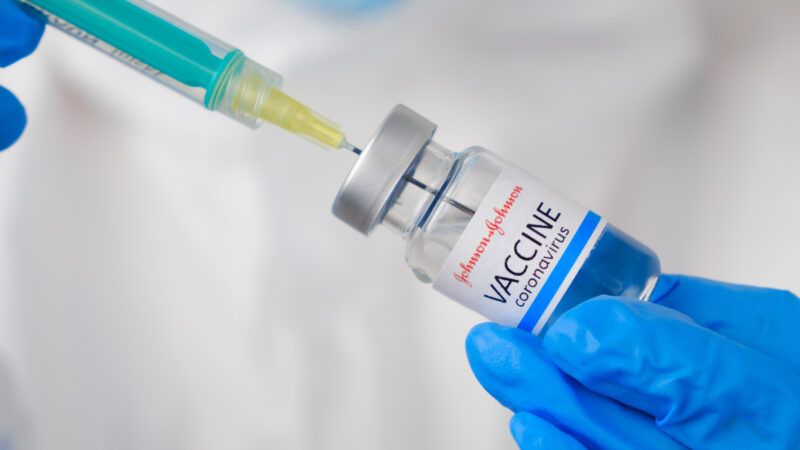
After a review of clinical trial data, the Food and Drug Administration's Vaccines and Related Biological Products Advisory Committee recommends that the agency issue an Emergency Use Authorization for drugmaker Johnson & Johnson's COVID-19 vaccine. That authorization would add a third COVID-19 vaccine to the already approved versions currently distributed by Pfizer/BioNTech and Moderna. If approved, the company says that it can deliver 20 million U.S. doses of its single-shot COVID-19 vaccine by the end of March.
The J&J vaccine uses a disabled cold virus that can enter human cells, but cannot reproduce, to deliver the gene for the coronavirus spike protein. This provokes the immune system to produce antibodies and other cells to fend off infection by the COVID-19 virus. The two earlier approved vaccines deliver messenger RNA (mRNA) for the spike protein encapsulated in tiny fat particles to get muscle cells to churn out viral proteins that then prime the immune system to fight the virus.
The FDA advisory committee reports that the J&J vaccine is 66 percent effective at preventing mild/moderate COVID-19 symptoms and 85 percent effective at preventing severe symptoms. Importantly, the vaccine was 100 percent effective at preventing hospitalizations and deaths from COVID-19 infections.
The J&J vaccine works after only one dose and does not need special refrigeration or other special handling. This contrasts with the FDA's current two-dose prescription for both the Pfizer/BioNTech and Moderna vaccines injected several weeks apart and their finicky ultra-cold refrigeration requirements.
Pfizer/BioNTech and Moderna have pledged to deliver before the end of March an additional 140 million doses of their vaccines over the 80 million they have already distributed. Together, and adhering to the two-dose regimen, that's enough to fully vaccinate 110 million Americans. Adding the 20 million single-shot doses of J&J's vaccine bumps that up to 130 million potentially vaccinated.
Let's assume that practice makes perfect and that the heretofore slow and bumpy rollout of the vaccination campaign is greatly sped up such that inoculations occur almost as fast as doses can be delivered. What would this extravagant thought experiment imply about how soon the goal of herd immunity might be reached?
In data scientist Youyang Gu's "path to herd immunity normality" calculations, he projects that it won't be until June 4, 2021, that the low 60 percent herd immunity threshold of 195 million Americans is reached. Actually, Gu now believes that the goal of herd immunity is a chimera and argues that the likely and reasonable objective is to significantly reduce COVID-19 deaths and hospitalizations so that life can return to normal. That will occur as COVID-19 becomes an endemic background infection with an annual death rate similar to that of seasonal influenza. (On the other hand, researchers are hard at work on universal coronavirus and influenza vaccines that could greatly reduce future misery and deaths from these viruses.)
So by June 4, Gu projects that 63 million Americans would be immune due to prior infections, 37 million would be immune due to both prior infections and vaccination, and 95 million immune solely as a result of vaccination. Overall, Gu projects that 135 million Americans would have been vaccinated by June 4.
So what happens if it happily turns out that something close to 130 million Americans are vaccinated by the beginning of April, instead of by June? Gu currently projects that 96 million Americans will have become immune due to COVID-19 infections by that date. Combining 130 million vaccinated people with 96 million people immune via infection very roughly yields 226 million Americans immune to the virus by April 1. Of course, the vaccination campaign will not go all that smoothly and there is double counting of people who have been both infected and vaccinated, but these crude calculations suggest that herd immunity, or at least, post-pandemic normality is close at hand.



Show Comments (45)