Moderna COVID-19 Vaccine 'Highly Effective,' Says FDA Analysis
Now we wait for the FDA to get around to approving it later this week.
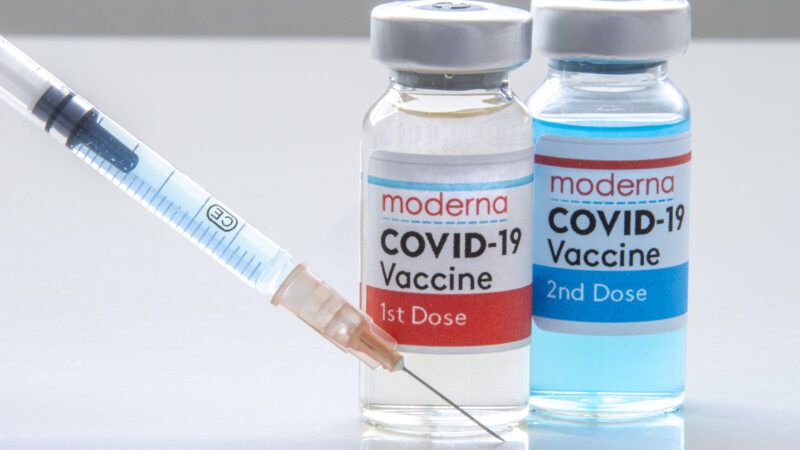
The COVID-19 vaccine from Moderna Therapeutics is 94.1 percent effective at preventing infection from the coronavirus that causes the illness, according to the Food and Drug Administration's (FDA) staff analysis. This efficacy rate was calculated based on the finding of 11 COVID-19 cases in the vaccine group and 185 COVID-19 cases in the placebo group. Additional good news is that 11 cases of severe COVID-19 occurred in the placebo group whereas none occurred in the vaccine group. Furthermore, the report notes that the vaccine was similarly efficacious for all "age groups, genders, racial and ethnic groups, and participants with medical comorbidities [like obesity and diabetes] associated with high risk of severe COVID-19."
The Moderna vaccine is based on the same messenger RNA (mRNA) technology used to develop the Pfizer and BioNTech vaccine for which the FDA just issued an Emergency Use Authorization (EUA) over the weekend. Therefore it seems highly likely that the Vaccines and Related Biological Products Advisory Committee, the outside panel of experts advising the FDA, will vote to approve this vaccine as well when the panel gets around to meeting this Thursday.
The reported side effects of the Moderna vaccine appear to be a bit more frequent than those of the Pfizer/BioNTech vaccine but resolve for most people after a day or so. In addition, the FDA report notes that the trial data are too limited to determine if it is possible for vaccinated people who nevertheless become infected and remain asymptomatic to transmit the virus to unvaccinated people. This is the case for the Pfizer/BioNTech vaccine, too.
For full efficacy, both the Moderna and Pfizer/BioNTech vaccines require two doses administered several weeks apart. The first inoculations with the Pfizer/BioNTech vaccine began yesterday. The federal government has contracted with Pfizer/BioNTech to purchase 100 million doses (enough for 50 million people) and 200 million doses from Moderna (enough for 100 million people).
The federal government is now trying to obtain an additional 100 million doses of the Pfizer/BioNTech vaccine sometime in the second quarter of 2021. Overall, that would be enough vaccine to protect 200 million Americans. That many doses would be sufficient to vaccinate most Americans over age 18, keeping in mind that the risk of dying from COVID-19 for people under age 18 is very low.
Two of the other vaccines—Sanofi and AstraZeneca—backed by the federal government's Operation Warp Speed have hit clinical trial road bumps that suggest that they may not be available (if ever) until the latter part of 2021. On the other hand, the clinical trial results from Johnson & Johnson's one-dose vaccine are expected to be available before the end of January. If the trial results turn out to be promising, the company will seek an EUA in early February and that would add a third vaccine to the U.S. arsenal. Johnson & Johnson has contracted with the federal government to supply 100 million doses before the end of the first quarter of 2021. That would then just about cover the whole U.S. population.


Show Comments (56)