Radiation Fantasies
Antinuclear activists have sounded the alarm over low-level radiation. A distinguished physicist separates fact from fantasy.
I find it ludicrous for anyone to claim that the required degree of containment for radioactive poisons will ever be achieved. And that opinion does not depend on the occurrence of major nuclear power plant accidents. Therefore, I am convinced that there should be no future permitted for nuclear power.
—John W. Gofman,
National Forum, Summer 1979
It is certain that ionizing radiation can induce cancer in humans. That is proven, even at very low doses now, by comparing the incidence of cancer among people irradiated at one dose (e.g., only by natural radiation) with people irradiated at higher doses.
—John W. Gofman,
Irrevy (1979), p. 61
The nuclear industry has been killing people every day for years in the form of cancer-leukemia deaths for the workers, and because we haven't declared the workers to be lepers, we let them procreate and thus they spread their radiation damage into the population at large.
—John W. Gofman,
testimony before California Assembly,
Apr. 1979
The decision to build and operate a nuke [nuclear power plant] is nothing less than premeditated random murder upon this and future generations.
—John W. Gofman,
Inquiry, Feb. 4, 1980.
Could John Gofman and other scientists who count themselves antinuclear activists, be right about the effect of radiation on human health? It is, clearly, a scientific issue, but people are now well aware that scientists can be found on both sides of the nuclear fence. What they may not be so aware of is that the community of scientists concerned with radiation health has arrived at a consensus on that subject that differs markedly from the ideas promulgated by John Gofman. And in evaluating his claims it is useful to know how that consensus has come about.
Science normally progresses by scientists from all over the world doing experiments, gathering relevant information, performing analyses, and eventually publishing their results in scientific journals. In addition, they gather occasionally at meetings to present their research results, with the opportunity for questions, counterarguments, and discussion. Since science deals with measurements and calculations that can be checked in other laboratories, and since tests can ordinarily be devised to shed light on the merits of competing ideas, it is generally not difficult to develop a consensus out of these interactions—anyone participating in the process can sense it forming as time goes on.
RADIATION CONSENSUS
On scientific issues that have an important impact on society, there is the additional mechanism of prestigious evaluation committees, and nowhere are there more of these than in the field of radiation health effects. There is the National Academy of Sciences' committee on Biological Effects of Ionizing Radiation (BEIR), funded (but with no attempt at exerting influence) by the Environmental Protection Agency. There is the United Nations Scientific Committee on Effects of Atomic Radiation (UNSCEAR), set up by the General Assembly in 1955. There is the International Commission on Radiological Protection (ICRP) and the US National Council on Radiation Protection and Measurements (NCRP), two independent agencies that have functioned continuously since they were set up over 50 years ago in response to problems that developed early in this century from overexposures to X-rays and to radium. (For more information on these groups, see "Scientific Radiation Committees," p. 25.) Britain has the Medical Research Council (MRC) and the National Radiological Protection Board (NRPB), and there are similar bodies in most industrialized nations of the world.
All of these groups are composed of eminent scientists in the field, with special attention to including diverse viewpoints and experiences. The BEIR Committee and the NCRP are composed entirely of American scientists, and of course the British groups are made up entirely of Britons. UNSCEAR and ICRP are truly international; UNSCEAR has a rotating leadership, with the most recent officers coming from Brazil, Belgium, Peru, Czechoslovakia, Poland, Germany, and India; and ICRP includes scientists from Canada, Sweden, Austria, France, Russia, Japan, Poland, the United States, and Britain.
All of these reputable groups continually study and update the evidence and frequently produce reports and recommendations. While there are many differences in detail among them, their estimates of health effects of radiation are generally in good agreement with one another and with the consensus of the involved scientific community.
LOW-LEVEL HYPOTHESIZING
How are these estimates of health effects derived? There have been many incidents in which appreciable numbers of people have been exposed to relatively high levels of radiation, and an increased incidence of cancer has been observed among them. There are the survivors of the A-bomb attacks on Japan; there have been several groups of patients treated with heavy doses of X-rays or of radium for various maladies; there were workers who were engaged in painting radium numerals on watch dials and who used their tongues to tip their brushes and thereby got radium into their bodies; there were miners exposed to high levels of radon gas in poorly ventilated mines; there were tuberculosis patients frequently examined by X-ray fluoroscopy, etc.
In all of these cases, we know the exposure doses in millirems (mrem; see "How Do We Measure Radiation?", p. 28), and the effects have been carefully studied. As a result, there is rather good information on effects of high-level radiation—doses above 100,000 mrem. The question is how to use this information to estimate effects of low-level radiation, of exposures less than 10,000 mrem.
The usual procedure is to use the "linear hypothesis," that is, to assume that effects are proportional to radiation dose, or in other words, that the risk per unit of dose is the same for all doses. To illustrate this, roughly a million mrem give a 15 percent risk of cancer; it is then assumed that 100,000 mrem give a 1.5 percent risk; 10,000, a 0.15 percent risk; 1,000, a 0.015 percent risk; and so on. Citizens of Harrisburg, Pennsylvania, received about 2 mrem from the Three Mile Island accident, so it is assumed that their risk of eventually getting cancer as a result is about 0.00003 percent (3 chances in 10 million). All of the above-named scientific groups consider this linear hypothesis to be a conservative approach, more likely to overestimate than to underestimate the effects of low-level radiation; that is, if anything, the risk per unit of dose is less at low dose than at high dose.
This is not just a claim meant to soothe people's fears about radiation. It is a judgment based on a wide variety of evidence. One of the more important considerations is our understanding of how radiation induces cancer. Our knowledge in this area is rather well developed and supported by extensive experimental evidence, and it indicates that low levels should be less effective than estimated from the linear hypothesis. There is also a considerable amount of direct experimental evidence on radiation-induced cancer extending to doses as low as 25,000 mrem. This evidence indicates that, if anything, the risk per unit of dose is less at low doses. There are many animal experiments, mostly on mice, that show this. The human data on Japanese A-bomb survivors and radium-dial painters give a strong indication in this direction, and no human data indicate the contrary. There is strong support from a comparison of lung cancer induced by radon among miners and in the general population. (Radon is a naturally radioactive gas to which all of us are continually exposed.) There is also evidence that the time required to develop cancer increases with decreasing dose, so that at low dose this time would be much longer than a normal lifetime. All of these matters have convinced the evaluation committees that the linear hypothesis is more likely to overestimate than to underestimate effects of low-level radiation.
One might ask why one can't get good direct evidence on effects of low-level radiation. The problem here is that, when effects are small, uncertainties in methodology can distort results and make them unreliable. For example, natural radiation is about twice the US average in Colorado, Wyoming, Utah, and New Mexico; citizens of these states get about 5,000 mrem more radiation in their lifetimes than the average US citizen. Since there are millions of people involved, statistical fluctuations are no problem, so this would seem to offer an excellent opportunity for learning about the effects of 5,000 mrem. What do the statistics show? They show that cancer occurs only about 70 percent as frequently in these states as in the nation as a whole; even leukemia, the type of cancer most closely associated with high-level radiation, occurs only 80 percent as frequently.
Should we then conclude that low-level radiation prevents cancer? No, because cancer is basically an environmental disease, and there are many environmental agents besides radiation that cause it. Exposures to these other environmental agents vary from place to place, and these variations could easily explain the low cancer rates in the Mountain states.
UNDUE ALARM
The news media have given heavy publicity to recent studies purporting to show that low-level radiation is more dangerous than indicated by the linear hypothesis. The most widely publicized study is by Mancuso, Stewart, and Kneale, who looked at workers in the government's Hanford Laboratory. Their results are in clear and direct conflict with data on the Japanese A-bomb survivors. But the A-bomb data are of much better quality; in addition, there are many holes in their reasoning. There have now been at least 15 published critiques of their techniques, and their work has been considered and rejected directly by the National Academy of Sciences committee (BEIR) and by the British NRPB. The ICRP has pointedly ignored it in issuing updates on radiation hazards. Yet the news media continue to publicize the Mancuso work while ignoring the critiques and rejections.
There have been a few other heavily publicized reports of work tending to enhance the importance of low-level radiation, but these have mostly collapsed. A preliminary study of workers at the Portsmouth Naval Shipyard in New Hampshire seemed to show an excess of leukemia (above the average rate for the United States), but when information on doses became available it turned out that relatively few of the victims had been exposed to radiation—there are many other cancer-causing agents in a shipyard environment. In testimony before the Senate Subcommittee on Health, chaired by Sen. Edward Kennedy, the two doctors responsible for the report drew back from most of their conclusions. When one of them nevertheless warned about inadequate safety standards for nuclear workers, even Senator Kennedy, no friend of nuclear power, became agitated. "I don't think we ought to be alarming families unduly," he exclaimed. "We have seen you repudiate two areas of your study and the National Cancer Institute has repudiated the third."
There were heavily publicized reports of excess leukemia in Grand Junction, Colorado, where uranium mill tailings were used in construction, but it was later found that the leukemia rates were the same for those who did and did not live in houses constructed with these mill tailings. There were reports of excess cancer in the vicinity of a plutonium plant near Rocky Flats, Colorado, but plutonium causes lung cancer, and there was actually a deficiency of lung cancer near Rocky Flats. Reports of excess cancer among soldiers involved in a Nevada bomb test and in the population living downwind from the Nevada test site throw no light on the problem because there is no good information about doses.
Now that all the dust has settled from these reports, the radiation health community has reached a consensus, represented by the statements of its various committees, that effects of low-level radiation are no different from what they were judged to be in the early 1970s. In numerical terms, the cancer mortality risk from 1 million mrem of radiation to a person's whole body is 18 percent according to BEIR, 12 percent according to UNSCEAR, and 10 percent according to ICRP. All of these groups acknowledge uncertainties in these estimates large enough to explain the differences among them.
People often ask how we can know the effects of the Three Mile Island accident when they will not occur until many years in the future. The answer is that we have measured the radiation exposures in mrem—these have already occurred. We then use our knowledge of the risk per mrem of exposure (as given in the last paragraph) to estimate the effects that will eventually occur.
One sometimes hears that radiation from nuclear plants is somehow different from natural radiation or from X-rays, but this is not the case. There are several different types of radiation from nuclear plants, but all of them also occur in natural radiation; and when radiation doses are expressed in mrem, all types are equivalent in terms of biological damage. Indeed, that is part of the definition of the millirem (again, see "How Do We Measure Radiation?", p. 28).
AND GOFMAN?
What, now, can we make of the claims of Dr. John Gofman about low-level radiation from nuclear power plants? Gofman is a physician and a nuclear chemist. As a graduate student in the early 1940s he codiscovered, with Glenn Seaborg, uranium-233 and went on to demonstrate its fissionability (it is one of three substances used for making atomic bombs and fueling nuclear power plants). In 1947, with a Ph.D. in nuclear/physical chemistry and an M.D., he was appointed to the department of medical physics at the University of California at Berkeley, where he specialized in research on coronary heart disease.
From 1963 to 1969 Gofman was associate director of the Lawrence Livermore Laboratory and directed the Atomic Energy Commission's new biomedical lab at Livermore. In 1969, he and a Lawrence Livermore colleague, biophysicist Arthur Tamplin, presented a much-publicized paper critical of the AEC's (and international) standards for allowable doses of radiation, although prior to this time Gofman had made such statements as: "A public stampede due to unfounded fears can lead to political and social disaster that might outweigh tremendously even the most pessimistic assumptions concerning radiation hazards."
That same year, 1969, Gofman left his post as director of the Lawrence Livermore Laboratory (he remained a research associate until 1973). He has throughout this time been a member of the UC Berkeley faculty, emeritus since 1973. He has emerged as a prominent antinuclear spokesman and currently spends three-quarters of his time working as chairman of the San Francisco-based Committee for Nuclear Responsibility.
THE CASE OF THE 32,000
Perhaps Gofman's best-known statement, dating back to the 1969 Gofman-Tamplin collaboration, is that if everyone in the United States were to receive the allowable dose of radiation there would be an additional 32,000 cancer fatalities per year. This is widely interpreted to mean that if we have a full nuclear power program there will be an extra 32,000 fatalities per year. The basis for this conclusion is that the internationally accepted maximum permissible dose to large populations is 170 mrem per year, and Gofman claims that this would cause that many fatalities.
If we use the risk estimates of BEIR, UNSCEAR, or ICRP given above, 170 mrem per year to each person would cause 3,500 to 6,000 fatalities per year in the United States, so Gofman is five to nine times higher than any of them. The BEIR committee studied Gofman's arguments, devoting six pages of their 1972 report to comments on them, and concluded that they should be rejected. His 32,000 extra fatalities should be no more than 6,000.
But this is only the beginning of the problem. Much more important is the fact that the 170 mrem per year limit has no relevance to the US nuclear industry. The Nuclear Regulatory Commission has a licensing requirement that no member of the public be exposed to more than 5 mrem per year. This applies to the few people who live closest to the plants; so as long as this requirement is fulfilled, there is no possible way for the average American to be exposed to more than about 0.5 mrem per year, which is 300 times less than Gofman's 170 mrem per year. His estimate of 32,000 extra fatalities per year is therefore too high by a factor of at least 5 x 300 = 1,500, making the maximum fatality rate from our nuclear program more like 20 per year. Actually, nearly all operating plants keep their radioactivity releases well below the maximum allowed, so the actual fatality rate would be even lower.
Gofman claims that government agencies use highly optimistic assumptions when they estimate the amount of radiation people are exposed to from nuclear plant operations. So let's consider, as an example, the assumptions of the Nuclear Regulatory Commission lying behind the requirement that no member of the public be exposed to more than 5 mrem per year. They assume that someone spends the entire year, day and night, at the point on the plant fence where radiation levels are a maximum. They assume that he gets no protection from being inside buildings—actually, this cuts exposures at least in half. They assume that he gets all his vegetables, meat, and milk from the nearby farm that gets the maximum radioactive contamination from the plant—actually these foods come from all over the country, so his radioactivity intake through food would be very much less than assumed.
Are the assumptions they make highly optimistic, as Gofman claims? The above are only a few examples; conservative assumptions, more likely to overestimate than to underestimate the radiation exposure, are made on any matter where there is appreciable uncertainty, and licenses are issued on that basis.
THRESHOLD TALK
Some scientists believe that very low levels of radiation are almost completely harmless, that is, that there is a "threshold" radiation dose below which there is essentially no harm to human health. Gofman argues correctly that there is no proof for such a theory (although there is also no proof that it is wrong). But then he goes on to give the impression that his opposition comes from proponents of the "threshold" theory. That is not the case. The threshold theory is not used by any government agencies or by any scientists publishing assessments of environmental hazards from nuclear power. They always use the linear hypothesis that the effects per unit of dose are the same at low doses as at high doses (this is also the theory Gofman uses).
As noted above, all of the scientific groups charged with assessing radiation effects assert that use of the linear hypothesis is more likely to overestimate than to underestimate the dangers, but nevertheless this theory is always used. This is another example of conservatism in nuclear regulatory processes and again is another counterexample to Gofman's charge that they are based on highly optimistic assumptions. When Gofman criticizes the threshold theory, he is beating on a "straw man."
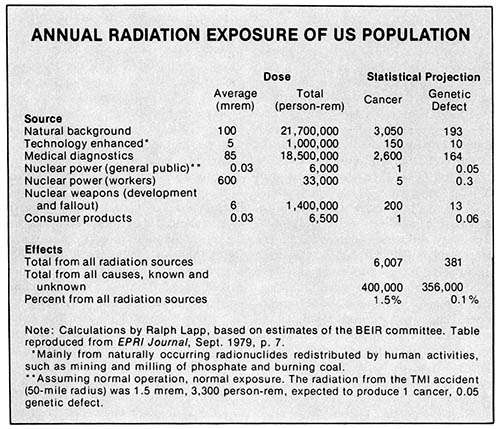
As an offshoot of his complaints about the threshold theory, Gofman usually points out that, if there is no threshold, even the small amounts of radioactivity released from nuclear plants are killing people. Actually, this point is generally accepted; we showed above that releases from a full nuclear energy program might cause up to 20 extra fatalities per year. But Gofman then exudes outrage at the idea that an industry is allowed to kill people. The trouble is that this outrage is very selective.
All industries cause fatalities among the public. The air pollution from coal burning, which is our only present alternative to nuclear energy, is estimated to be killing at least 10,000 Americans per year, at least 500 times the toll from the nuclear radioactive emissions Gofman complains about. Other forms of energy also take their tolls—oil causes air pollution and fires; natural gas kills by explosions, fires, and asphyxiation; hydroelectric dams can break with truly disastrous results; geothermal energy has very serious air and water pollution effects; and so on. Even solar energy, if it were available as an economically competitive source of electricity, would require the use of vast amounts of steel, which is made by burning coal and thus causes murderous pollution problems. All of these competitive energy sources kill far more than 20 people per year in the United States. For the solar energy option, a typical estimate is about 1,000 fatalities per year.
Chemical, paper, metal, and many other industries are also well-known polluters. But people evidently want the benefits of manufacturing and energy-producing processes. People use the roads as motorists, pedestrians, and cyclists even though there are fatalities. If they continue to do so in spite of the risks—motor vehicles annually kill 40,000 of their users as well as 10,000 pedestrians and 1,000 bicycle riders—is it so odd to think that they might be willing to accept the risks associated with coal burning, oil, natural gas, solar energy production, and industrial processes—and the much lower risks associated with nuclear power generation?
PLUTONIUM SCARE
Aside from his fear-mongering over the emissions of low-level radioactivity from nuclear plants, Gofman's biggest issue has been the danger from plutonium toxicity. His arguments are set out in two papers he wrote and distributed privately in 1975 (they were never published in a scientific journal).
In his first paper, Gofman introduces a new method for estimating the toxicity for plutonium, making several blatant errors. For example, he uses his own crude estimate of the area of the bronchial surfaces in humans, but the estimate is 17 times smaller than the generally accepted area. He assumes that dust settling in the bronchi of cigarette smokers is cleared away thousands of times more slowly than in nonsmokers in spite of the fact that there are direct experimental measurements of these clearance times which show that there is no large difference between smokers and nonsmokers in this regard. (Smokers compensate for loss of normal clearance mechanisms by extra coughing and mucus flow; if they actually cleared their bronchi as slowly as Gofman assumes, they would die of suffocation.) He uses the same erroneous procedure that made his estimates of effects of radioactivity from nuclear plant emissions so high, thus increasing effects by another factor of five. And he pulls other skulduggeries. As a result, he concludes that the risk from plutonium inhalation is about 1,000 times greater than the value generally accepted among scientists who have concerned themselves with this issue.
At least five devastating critiques of this paper have been published, and I have never encountered a radiation health professional who takes Gofman's risk estimate seriously. It has been completely ignored by ICRP, UNSCEAR, and BEIR, which have since published reports reviewing serious evidence on these matters. It has also been completely ignored by NCRP and the British MRC and NRPB groups, which had previously published reports on plutonium toxicity with conclusions in the usual range and have not seen fit to amend them in the light of Gofman's thousand-times-higher estimate.
In his second paper, Gofman develops an argument based on a statement that sounds reasonable—that the nuclear industry cannot achieve better than 99.99 percent containment of its plutonium. He doesn't very lucidly explain that he interprets this to mean that 0.01 percent of all plutonium will be dispersed into the atmosphere as dust particles so fine that they will remain suspended in the air for at least several days (it is only these tiniest of particles that can get past the body's defenses into the lungs, where they can do damage). Ordinarily, only a minute fraction of lost material escapes in this form, so it is highly deceitful to assume, as Gofman does, that all of it will.
Present federal regulations on plutonium handling require that no more than one part in a billion be released as airborne dust (of any particle size), and all plants that handle plutonium have been successfully staying within these limits for several years—this is 100,000-times-better containment than Gofman assumes.
There are several reasons why it is not difficult to keep releases so low. Plutonium is a heavy solid material, not well adapted to floating around in the air. When in powdered form, the particles tend to stick together, to their container, and to any surface (e.g., walls) they contact. Directing an electric fan at an open box of powdered plutonium would result in very little of it becoming airborne. Quantities of plutonium are not large—the total amount in existence would fit into a household closet—so it is not difficult to exercise great care in handling it. Any box in which plutonium is being handled or stored is always inside another "box" that would contain it if the inner box should break, and the entire system is inside a sealed building (or truck) from which air can only exit through filters capable of removing plutonium dust.
The only creditable source of appreciable releases of plutonium is in accidents, and in the worst incident of this sort to date—a 1957 fire in a Rocky Flats, Colorado, plant—only about 1/100,000 of the plutonium inside escaped as airborne dust. This was before many of the modern safety precautions were instituted. In a 1969 fire there in which a production building was destroyed with $50 million damage, only about 1/10,000,000 of the plutonium escaped as airborne dust. In these escapes of airborne dust, only a small fraction of the material would be in particle sizes small enough to be dangerous, and of course, only a tiny fraction of all plutonium is involved in accidents.
Another misrepresentation of Gofman's second paper is that he bases his estimates on 400 million pounds of plutonium. None of our present electric power is produced by fast-breeder reactors, the type that use the most plutonium. But even if all of it were, there would be a total of only 2 million pounds of plutonium in the reactors. Here we have a 200-fold overestimate of the possible dangers, which is combined with his 1,000-fold exaggeration of the toxicity of plutonium and his even more gross exaggeration of the escape probability. Thus, the conclusion of his second paper, that there would be 20,000 fatalities per year from plutonium in a full-scale nuclear power economy, should be reduced to something less than one fatality per century.
John Gofman was at one time a respectable scientist. He is an excellent speaker, full of anecdotes, often about what he says some former government bureaucrat or some corporation executive told him in private conversation some time in the distant past—there is no shortage of poorly informed people of this type in our society, and quoting them out of context can make them seem even more stupid and insensitive than they are. He is a charming person in every way; after every conversation between us, I have come away liking him. But before you accept his fantasies about the dangers of radiation, I hope you will consider some of the information presented here.
Bernard Cohen is a professor in the Department of Physics and Astronomy at the University of Pittsburgh. He has worked at Oak Ridge National Laboratory and as director of Scaife Nuclear Laboratories. He has held office in several professional associations, among them chairman of the American Physical Society, Division of Nuclear Physics, and is chairman-elect of the Division of Environmental Sciences of the American Nuclear Society. Among his books is Nuclear Science and Society (1974).
SCIENTIFIC RADIATION COMMITTEES
X-rays were discovered by William Roentgen in 1895. In the years that followed, the medical use of X-rays, for both diagnosis and therapy, became widespread, and knowledge of their cancer-causing and genetic effects began to accumulate. In the 1920s several agencies—the International Commission on Radiological Protection (ICRP) and, in the United States, the National Council on Radiation Protection and Measurements (NCRP)—were formed to provide guidelines for occupational exposure to radiation (for example, in the administration of radiation treatment for cancer). Both groups were made up of scientists; the ICRP, mostly of radiologists.
Following World War II, with expanding nonmilitary uses of nuclear energy on the horizon, two other groups joined the ICRP and NCRP: the National Academy of Sciences set up a Committee on the Biological Effects of Ionizing Radiation (BEIR), and the United Nations formed a Scientific Committee on the Effects of Ionizing Radiation (UNSCEAR). All of these groups are expected to review and assess the scientific literature on the effects of exposure to various levels and types of radiation and on this basis to provide guidelines for permissible exposures of the public.
When the National Council on Radiation Protection was first established, it included one member from each of the concerned professional (mostly medical) organizations, plus one member from the National Bureau of Standards. Over the years it has expanded to include 75 members serving six-year terms, with new members elected by the present council membership from nominations by various groups and individuals. In addition to council members, close to 350 other scientists serve on the NCRP committees that review studies and draft proposed recommendations that are submitted to the council. The NCRP is supported by annual membership dues and foundation and government grants.
The ICRP membership is similarly self-generating, with the original membership chosen by the International Congress of Radiology, a professional association. Members of the BEIR committee are chosen by the officers of the National Academy of Sciences, who are in turn elected by the academy's membership of about 1,000 scientists from all fields of science. UNSCEAR members are also scientists, appointed by their governments.
—Marty Zupan
HOW DO WE MEASURE RADIATION?*
Ionizing radiation produces a stream of fast-flying particles or waves that come from the nucleus of unstable atoms or man-made machines. The conventional unit of measurement of the amount of energy deposited in living tissue is the radiation absorbed dose, or rad (1 rad equals 100 ergs per gram of tissue).
Radiation comes in several basic types: alpha, beta, and neutron particles and gamma and X-rays. Since these vary widely in their ability to penetrate tissue and alter molecular structure through ionization, the rad is weighted for the increased biologic effect of particles (that is, a rad of exposure from alpha particles produces a greater biologic effect than a rad from X-rays). This weighted measure of exposure is a rem, and it is independent of the type of radiation (that is, a rem of exposure would be expected to produce a constant biologic effect, regardless of the type of radiation).
In discussions of low-level radiation, dosage is often expressed in millirems (mrem), which equals 1/1000 of a rem. For example, the average person in the United States receives 85-100 mrem per year from medical sources and an additional 100 mrem per year from natural sources, such as from the radioactivity in soil and building materials, cosmic radiation, and the natural radioactivity in the human body itself.
The person-rem is a measure of population exposure rather than a measure of individual exposure. It is defined as the added doses to each of the exposed individuals. The underlying assumption is that of a linear relationship between dose and effect. For example, 100 persons exposed to 2 rem each would be 200 person-rem, and the ultimate effect is assumed to be identical to one person receiving 200 rem, which would also represent 200 person-rem.
—Leonard Sagan
* Reprinted from "Radiation and Human Health," by Leonard Sagan, EPRI Journal, Sept. 1979.
This article originally appeared in print under the headline "Radiation Fantasies."


Show Comments (0)