Containing Nuclear Waste
Safe disposal of radioactive waste is often called the Achilles' heel of nuclear power. How far along are we on the road to solving this problem?
It may be hard to believe given the current state of public disinformation about nuclear power, but the vast superiority of waste disposal is one of the salient advantages of generating electricity from nuclear energy. If that sounds crazy, consider the following five well-kept secrets:
• It is utterly untrue that no method of disposing of radioactive waste from nuclear power plants is known.
• It is utterly untrue that nuclear wastes must be guarded for thousands of years.
• The real issue is not whether nuclear wastes pose hazards but whether their disposal offers a significant advantage in safety, public health, and environmental impact when compared to the disposition of wastes from fossil-fired plants (let alone industrial wastes in general).
• Much of the result of such a comparison is attributable to two simple statistics: for the same power output, nuclear wastes are some 3.5 million times smaller in volume; and in duration of their toxicity, the advantage ranges from a few percent to infinity.
• Nuclear power does not add any radioactivity to the earth; on the contrary, it reduces the radioactivity that Mother Nature would otherwise be producing.
THE BIG WASTE PICTURE
The Environmental Protection Agency estimates that some 385 million tons of industrial wastes, 10-15 percent of them hazardous, were produced in the United States in 1980. Until recently, it was standard practice to dispose of such wastes by dumping them in unlined surface ponds, unsecured landfills, sewers, or deepwells; by burning them in uncontrolled incinerators; or by spreading them on roads. Some was and is disposed of—ask not how—by gypsy haulers and moonlight dumpers.
When strict federal regulations—requiring containment and monitoring of hazardous wastes—went into effect earlier this year, the EPA estimated that only 10 percent of existing disposal sites would meet the new standards. Some of the other 90 percent would never make the grade; the rest would require an interim period of some years to come up to par.
The volume of toxic wastes generated in a single year is more than 70,000 times larger than the volume of nuclear wastes accumulated by nuclear power plants since the beginning of the nuclear era 24 years ago. Their toxicity is retained for centuries in the case of the more stable chemical compounds such as polychlorinated biphenyls (PCBs). Elemental toxins such as cadmium, beryllium, or arsenic remain toxic forever—unless, of course, they are luckily radioactive, so that they will disintegrate by radiation. Otherwise they will be around long after the last atom of radioactive potassium 40 in Ralph Nader's blood (halflife 1.2 billion years) has decayed.
The point of all this is not to say that waste disposal is a mess anyway, so it doesn't matter if we make the situation a little worse with nuclear wastes. This is simply the context within which any waste disposal problem, nuclear or not, must be considered.
In fact, however, nuclear wastes would not make the situation worse but would eliminate part of the problem. A very significant part of industrial wastes can be traced to the generation of electricity in power plants that burn fossil fuels. These plants, particularly coal-fired ones, produce voluminous, dangerous, and persistent wastes. Replacing them with nuclear power plants would reduce electricity-generation wastes to a minuscule volume, disposable by far safer, cleaner, and healthier methods.
Still, while coal wastes take a shockingly large toll in deaths and diseases, there is one thing worse than coal, and that is no coal. Researchers at the International Institute of Applied Systems Analysis in Laxenburg, Austria, analyzed data from 150 countries for the last 75 years and found that coal saves more lives than it takes. So the purpose of comparing nuclear and coal wastes is not to fight coal but to show the millionfold advantage of nuclear waste disposal over the waste disposal that prevails now.
And what if we were to turn to some other alternative? There is no such thing as large-scale power generation without wastes. The nearest to it is hydropower, but even that produces wastes in the manufacture of its machinery, in the construction of its dams, and in the energy conversions for both. In any case, since most hydropower sites in the United States have already been taken, hydropower does not really offer much of an alternative for large-scale power generation.
Even solar power is not without wastes. In fact, construction of its collectors produces about 1,000 times more wastes than for any other electric power conversion—a direct consequence of its diluteness. According to figures released by the federal government's Solar Energy Research Institute, the construction of a 1,000-megawatt (MW) solar plant would need 1,000 times more materials than would a conventional plant of equal capacity, whether fossil-fired or nuclear: 35,000 tons of aluminum (at an energy cost of 75 million BTUs per ton), 2 million tons of concrete (at 12 million BTUs per ton), 600,000 tons of steel (at 56 million BTUs per ton), 75,000 tons of glass (at 18 million BTUs per ton), etc. If the 1,000 MWs were not produced centrally but were distributed over many small, domestic units, the imbalance would be worse—for the same reason that a large central bakery wastes less flour per loaf than 10,000 housewives who bake one or two loaves each.
Moreover, unlike conventional sources of electricity, solar power is not self-sustaining: it cannot now, or in the foreseeable future, produce the electricity needed to manufacture its own components. In practice, therefore, the wastes involved in producing solar power are merely pushed off to nonsolar generation.
COAL WASTES BY THE TON
All the tons of coal that go into America's power plants must come out as tons of waste with not a single ounce forgiven. And how much coal goes into America's power plants? Last year it was 566 million tons. Or 1,077 tons a minute. Or about 18 tons since you began reading this paragraph. And that's just the coal going in; the wastes coming out are more than twice that weight: a power plant consumes not only coal but also atmospheric oxygen (and a little nitrogen) to produce its wastes.
Somewhere near you there is a coal-fired power plant, perhaps a big one with 1,000 MWs or greater capacity. The coal comes in by unit train, with cars carrying 100 tons each. Each car is grabbed by a rotary dumper that turns it upside down to empty its load onto transporters and then puts it back onto the rails. It dumps one 100-ton car every two minutes for much of the day shift—twice as fast if the cars have the new couplings that allow the dumper to handle them without uncoupling them from the train.
100 tons a minute! And all those 100 tons must end up in one of two places: a landfill or the atmosphere. There is nowhere else for it to go.
The wastes leave the plant at several places: the stack disgorges gases and particulates; the bottom ash (from the furnace) and the sludge from the scrubbers go into lined settling ponds, where the water evaporates and the dry residue is then taken to landfills; most of the fly ash is captured by electrostatic precipitators or by mechanical filtering in baghouses (the hot gases are forced through bags of fairly finely woven textile) and is trucked to landfills.
In a 1,000-MW unit, solid wastes are produced at the rate of some 30 pounds per second.* They include 19 toxic metals (such as arsenic), carcinogens (such as benzopyrene), and as reported in Science in January 1978, some mutagens.
They are also radioactive, as are the stack emissions—up to 50 times more than the routine emissions from a nuclear plant. If coal-fired plants were subject to the regulations of the Nuclear Regulatory Commission, most would have to be shut down for exceeding radioactive limits. The radioactivity is due to the uranium, thorium, pollonium, radium, and other radionuclides in the coal (some of them are soluble in water and chemically active). It is not the radioactivity that makes coal wastes dangerous, however; even if 50 times greater than that from nuclear plants, it is still minute. The point is simply to note a comparison of which most people are utterly unaware.
All these goodies are dumped in landfills, where nobody monitors them, and their health effects appear only after they have been leached out of the dump. Except for the radioactive isotopes, the half-life of the toxic elements like arsenic or mercury is infinite. Their volume is stupendous: The sludge from the scrubbers alone is expected to cover 240,000 acres to a depth of six feet by the end of the century.
Yet the solid wastes are much the smaller problem. The real health hazard is the wastes disgorged by the stack. Per 1,000-MW unit, they include:
• 600 pounds of carbon dioxide per second—not toxic, but possibly responsible for climatic changes.
• 30 pounds of sulfur dioxide (and some sulfur trioxide) per second—linked to lung, heart, and bronchial diseases by striking correlations (though a direct cause-effect relationship need not necessarily follow).
• As many nitrous oxides as 200,000 automobiles running simultaneously—producing photochemical, smogs and recently linked (via nitrosamines) to cancer in urban areas.
• Particulates—the ones that get past the precipitators and other filters because they are too small are also too small to be held back by the filtering mechanisms of the human body, and they reach the bronchi and the lung. Even if the precipitators are 99 percent efficient, 18 pounds of this fine stuff comes out of the stack every minute.
The carcinogens, mutagens, and toxins (or for that matter, the radioactivity) of these particulates are not tied to size. They are present to the same degree as in the bottom ash and fly ash, which is so abrasive that if used in a jet it can cut metal. Some of it is deposited in people's lungs.
That is the waste "disposal" we have now.
The results should not be surprising, yet they come as a shock to most people. A report prepared by the Office of Technology Assessment of the US Congress in 1979 estimated the number of premature deaths due to air pollution attributable to coal combustion:
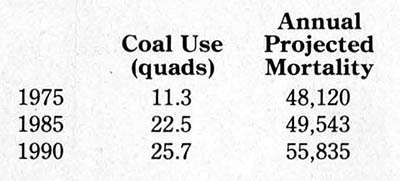
These numbers will not startle anybody who has been studying the public-health effects of power generation. What is startling is the way the national news media have ignored such matters in their coverage of the debate over nuclear power.
The figures are the best that are currently available, based on recent work at Brookhaven National Laboratory. Though they are carefully calculated from measured correlations with sulfate exposures in each 20-by-20 mile square of a grid covering the United States, they are not certain but probabilistic values subject to a statistical range of error and subject also to assumptions about future coal usage.
Electric power generation now accounts for about 81 percent of coal use. Its share of the cost of coal waste disposal into the atmosphere is some 39,000 premature deaths per year. That does not include the human costs from waste disposal in landfills, nor the other human costs of coal (deaths and injuries in transportation, industrial diseases, and accidents in the mines, all of which are vastly greater than for the uranium needed to produce the same energy.) Against this background, what is currently feasible in the technology of nuclear waste disposal is a largely unrecognized blessing.
NUCLEAR POLITICS
A nuclear reactor's fuel rods, which contain uranium oxide pellets, are replaced after about three years of service. The reason for replacement is not that their energy has been used up but that the fission products block the flow of neutrons necessary for an efficient chain reaction. These "spent fuel" rods are "hot" not only in the sense that they are radioactive; they are also thermally hot. They are cooled for some time in pools of water at the site of the power plant to let the short-lived components die away.
(The short-lived components are the dangerous ones, since their short life is spent in radiating away their energy more intensely. They also include some products that are dangerous for additional reasons—for example, iodine 131, with a halflife of 8 days, which can be trapped and retained by the thyroid gland, where it may give rise to cancer.)
After the fuel rods have been in the cooling ponds for some six months, they should be taken to a reprocessing plant, where they are cut up into short pieces and dissolved in nitric acid, in order to extract the remaining fissionable uranium and the plutonium formed in the rods and to recycle it into fresh fuel. (Yes, plutonium is formed in the rods of conventional light-water reactors, too. As much as one-third of nuclear power is generated from plutonium now, before the advent of plutonium breeders.)
This is not, however, what is actually happening in America now. President Carter prohibited reprocessing, and a plant at Barnswell, South Carolina, built to perform it, lies idle. Consequently, the fuel rods have been piling up in the cooling ponds, and power plants are running out of space. This in itself is not a fatal problem. In fact, it may be indirectly beneficial in that utilities are exploring above-ground, air-cooled facilities for storing the fuel rods—a method that has been proven safe in use in Canada and Europe and that promises to be far less expensive than the present water cooling.
The more lamentable aspects of the ban on reprocessing are that, amidst worries about increasingly scarce energy supplies, quadrillions of BTUs locked up in these fuel rods go untapped; and amidst worries about nuclear proliferation and terrorism, it is proposed that this energy be wasted in a "throw-away cycle" by burying these rods in tombs, until the whole country is littered with little plutonium mines in honor of President Carter's nonproliferation policy.
All of this, however, is merely politics. US policy in this area is not based on any technological constraints. Reprocessing technology is not only well developed but in commercial operation in Britain (Windscale), France (La Hague), and the USSR (location undisclosed, but quite likely in Dimitrovgrad on the Volga). All three countries also have full-sized breeder reactors on line in the public power net, and reprocessing is particularly advantageous for breeders. Two other countries, Germany and Japan, now send their spent fuel to France for reprocessing, but both have far-advanced plans for reprocessing plants (as well as for breeder reactors).
After reprocessing, with most of the uranium and virtually all of the plutonium (more precious than gold) extracted chemically from the acid, there remain high-level wastes. These are responsible for 99 percent of the radioactivity but amount to only 1 percent of the volume.
The high-level wastes are then solidified and sealed into a permanent leach-resistant medium such as borosilicate glass or ceramic or basalt, which is then itself sealed into a permanent container such as a stainless-steel or ceramic or cement canister. After some years of cooling in interim facilities, the canisters can be surrounded with an absorbent mineral filling and then buried, retrievably, in stable geological formations. All of these points need further explanations, and I will return to them. But first, two outstanding features of these wastes should be noted: their incredibly small volume, and their temporary toxicity.
Even though the high-level wastes are diluted with twice their own volume of inert materials as they are solidified into glass or ceramic, the grand total of these wastes for a 1,000-MW unit amounts to no more than two cubic meters per year—a volume that would comfortably fit under a typical dining room table. A coal-fired plant of equal capacity produces some 10 tons of waste—not per year, but per minute.
As for toxicity, the wastes will not have to be guarded for thousands of years. In 500 to 1,000 years (depending on how completely the transuranics are recycled at the processing plant and also on the concentration of the comparison ore), their radioactivity will have decayed to below that of the ore they originally came from. In the long run, nuclear power does not add any radioactivity to the earth; on the contrary, some of the energy that would have appeared as radioactivity of the ore, had it not been mined, will have been used up to give people light and warmth.
DISPOSAL DETAILS
Now let us return to some of the technical details of disposal.
The stainless steel canisters holding the wastes solidified in glass or ceramics will be 1 foot in diameter and 12 feet long. Before going into their ultimate repository, they will be stored for an interim period of about 10 years in air-cooled vaults or water basins. The only place in the United States where this is now being done (as a pilot project, because there are no commercial wastes yet ready to undergo this treatment) is the Idaho Chemical Processing Plant, where underground concrete vaults are used.
The advantage of delayed burial is a great easing of the cooling problem. The heat generated by the wastes will have died down to less than 3.5 kws per canister, or less than is released by the typical home laundry dryer.
The canisters can then be buried deep (1,500-1,800 feet) in stable geological formations, where, separated by about 10 yards (to provide cooling air flow and access for possible retrieval), they would take up a tiny area as waste disposal sites go: even if 100 percent, instead of 11 percent, of US power capacity were nuclear, the annual increase would amount to less than 100 acres.
A typical geologically stable formation is a salt formation, of which the United States has many. The existence of the salt shows that no water has been present for as long as the formations have been there (some 100 million years), or they would have dissolved. And if water were to get in after all, the salt would seal up and prevent more water getting in.
SO WHAT'S THE TROUBLE?
It should be clear from this brief account that there are no major engineering problems in disposing of nuclear wastes in a manner whose safety is unrivaled by any that can be applied to other wastes—in particular, to the fossil wastes whose high costs in public health could be eliminated by switching to nuclear power. Why, then, is the perception widespread that waste disposal is the problem holding up nuclear power in the United States? The answer is that no method of disposal has been adopted and legalized by the bureaucrats and politicians in Washington.
It is true that the need for permanent waste disposal is not very pressing right now. But the fact is that until people are made aware of the technical possibilities, they can be expected to continue to oppose in large numbers the present operation and new construction of nuclear reactors—at the expense of many lives each year.
Not only are there no major problems; there is even one case giving 1.8 billion years of experience with nuclear wastes. In Oklo, located in Gabon, Central Africa, five natural nuclear reactors were in operation for half a million years 1.8 billion years ago, producing two tons of plutonium and five tons of fission products. Almost all of the plutonium has now decayed, and the "wastes" have not budged for 1.8 billion years.
Disposal is not without minor problems, however, most of which are of the "Couldn't it be done even better?" type. There are a few who believe that burying the wastes below the sea bed would be better than on land and fewer still who would melt them into the arctic ice cap. But there is ongoing investigation of encapsulation substances and burial sites.
France and Britain have done a good deal of work with borosilicate glass as the material in which to immobilize radioactive wastes. Since 1978 the French have been operating a small glass plant at Marcoule. Yet there are reasons to believe that ceramics are better, and they may yet be adopted in the United States. Meanwhile, scientists led by Prof. A.E. Ringwood at the Australian National University in Canberra have developed and patented the manufacture of artificial rock that will so mesh with the crystalline structure of the waste that it will immobilize it for up to two billion years. (How do they know? From the geology of stable isotopes: radioactivity is a nuclear phenomenon and cannot affect chemical or macrophysical behavior.)
And at the Idaho National Engineering Lab (INEL), high-level wastes from the reprocessing of spent fuel rods from the navy's nuclear-powered submarines have been incorporated into a curious material developed by INEL scientists. The material looks like basalt, has the same physical properties as basalt, and has a very similar chemical constitution (it is just a bit richer in iron)—all for a very good reason: it is basalt. By slowly cooling artificial lava, the scientists at INEL have produced synthetic basalt—with the powdered nuclear wastes a part of it. The resistance of basalt to heat, moisture, leaching, weathering, earthquakes, and all other natural furies has been total, although admittedly experience is limited to about 200 million years.
As to the canisters holding solidified wastes, alternatives to stainless steel being actively considered include: copper; an alumina-zirconia ceramic favored by the French for its high chemical insolubility; and artificial sapphire, which has been used in Sweden to produce a full-scale canister that can last for millions of years, because nothing short of diamonds is hard enough to cut it open. In addition, geochemist Rustum Roy, writing in the April 1981 issue of Technology Review, predicts that "in the long run, concrete containers will no doubt be actively considered.…concrete's extremely low cost, convenience of fabrication in situ, and ease of sealing are simply unmatchable." Roy notes that existing opposition to concrete "has been based on unfamiliarity with current concrete technology (including amateur responses based only on the familiar concrete used in highways)."
Recently, scientists working on solving the nuclear waste disposal riddle have also come to believe that in certain instances, depending on the immobilizing and encapsulating materials used, it would be a good idea to add an "overpack" before long-term burial of waste packages. Consisting of mineral substances such as bentonite and quartz (a mixture under investigation by the Swedish), this overpack would protect the canister from any corrosives in the repository environment and would shield that environment from any escaping radioactivity.
Finally, some researchers are reconsidering salt beds as the repository site. According to Roy, recent findings indicate that salt is more corrosive and contains more water than long believed, so the Department of Energy and European agencies are exploring the possibility of other rock types, including shale, basalt, and granite.
The details of these alternatives make for fascinating reading. But given the current state of public opinion, one begins to feel like a person arguing the relative merits of this versus that anaesthetic at a time (only 120 years ago!) when people were convinced that to ease the pain of amputation there was nothing better than making the patient drunk and, if he could stand it, hitting him over the head with a club.
LOWER-LEVEL WASTES
Although the high-level radioactive wastes from spent fuel rods have been the greatest concern of those worried about nuclear power generation, there are other radioactive byproducts of the process also. There are low-level wastes, for example—usually defined as those with less than 10 millicuries per kilogram. They consist of discarded workers' gloves, paper, plastic, textiles, glass, etc., that have been or may have been contaminated with radioactive isotopes and otherwise resemble normal household or industrial waste. These account for only 1 percent of the radioactivity of all wastes, but for 99 percent of the volume. Only about 0.01 percent by weight of this waste is actually radioactive.
This material is put into drums and disposed of under controlled and monitored conditions in a repository a few feet underground, with asphalt flooring and other methods of ensuring their isolation—in the United States. Many, perhaps most, other countries using nuclear power simply dump these drums into the sea, which is no great abuse, for it is probably negligible compared with the total radioactivity of sewage and other wastes dumped into the oceans.
Radioactive waste is also generated in the nuclear fuel cycle in the processing of uranium ore. Tailings near mills that extract uranium oxide from uranium ore have a relatively high concentration of U 238 and thus form an open source of radon gas.
When a nucleus of uranium disintegrates by natural radioactivity, it may turn into one of the fission products that itself turns into radium, which begets radon, which begets a further chain of unstable elements ended by a stable lead. The most dangerous in the chain are radon and its daughters, which are highly radioactive, and as gases, have easy entrance into the human body by inhalation. Radon is, in fact, the most serious radioactive health hazard in the natural environment, causing some 10.000 cancer deaths per year in the United States according to estimates by the United Nations Scientific Committee on Effects of Atomic Radiation. Radon is also the main cause of lung cancer among uranium miners. (Although radon is shortlived, it is constantly being replenished by its grandmother uranium, which has a halflife of 4.5 billion years.)
The radon produced by uranium mill tailings was used by R.O. Pohl a few years ago to claim that nuclear power generation is more hazardous than coal-fired power. Even assuming, however, that the tailings would simply be left lying around and houses built on them (as has happened in some cases), Pohl acknowledged that this source of danger would have to accumulate for 80,000 years before it overtook the fatalities due to coal-fired power. That puts it well beyond the next ice age, and makes the calculations so bizarre that it no longer matters much whether they are right or wrong. In fact, though, coal turns out to be worse even in this freakish issue of what today's mining will do to radon emissions over the next couple of million years.
Uranium is one of the elements that is ubiquitous; it is mined only where its concentration is very high, but it is present virtually everywhere, and it produces radon everywhere. Radon is an inert gas that does not react with other chemicals in the ground but diffuses upward and is diluted in the atmosphere. When a hole is dug into the ground for any reason at all, the flow of radon into the human environment is increased, and this aspect of coal mining alone might be enough to defeat the theory, but there is more.
Coal contains an average of one part per million of uranium, which is released to the environment as a source of radon. Although the hazard is quite small, it is 1.000 times greater for a coal-fired plant than from the wastes of a nuclear plant of equal power. The reason is not that the nuclear plant uses less uranium (it uses incomparably more), but that in producing electric power it prevents most of its uranium fuel from turning into the radon that it would otherwise have produced. (The uranium used for nuclear power is that close enough to the surface to be mined, which is also close enough to the surface to release radon to the human environment whether it is mined or not.) What it produces instead is also radioactive, but the total dose to the environment is ultimately smaller than if the uranium had been left to decay on its own.
While the fatalities chargeable to this year's mill tailings will be drawn out over the next 80,000 to 2 billion years (with one fatality per year in the "worst" of these years), the radon trapped by buildings, especially unventilated ones, due to the ubiquitous uranium in its walls and foundations can more than double the outdoor background radiation, which itself is some 1,000 times higher than the average US resident gets from nuclear plants. This radon enters the lungs of the building's occupants, not in 80,000 years, but here and now. If your room is not well ventilated (for example, in order to use less energy for heating or cooling it) you may well be breathing it as you are reading these lines.
Any energy facility must, at a certain age, be taken out of service. The reservoir behind a hydroelectric dam will eventually silt up, and a fossil-fired plant has a life of about 30 years. Nuclear plants are licensed for 40 years and may have to be decommissioned after that time or earlier. In a sense, this is a case of waste disposal, too, but again one that is not insurmountable.
Some parts of the plant will have become radioactive. Surface contamination can be removed by chemical cleaning or sandblasting (as has been done in Chalk River, Canada), but the induced radioactivity bound within the material of the pressure vessel, for example, cannot be removed in this way. For the induced radioactivity of steel, the dominant source is cobalt 60, and it would take 50 to 100 years before it died down to a level at which the massive pressure vessel could be dismantled without special precautions.
The three ways of decommissioning a reactor are mothballing (blocking the entrances by pouring them shut with concrete and instituting some other security measures), entombing (burying the plant under a hill of earth), and dismantling by remote control. All three methods have been tried in practice and are technically feasible, so that the choice, when the decommissioning age is reached, is dictated by economic considerations.
Since 1960, 65 reactors have been decommissioned in the United States, including 5 power reactors, though none of the large units (1,000-MW or more) have yet been dismantled. Since the reactor building is only a small part (less than one-tenth) of the usual 25-acre site of a nuclear plant, mothballing would not prevent a new reactor being used on the same site, and one of the favored options is mothballing for 100 years followed by straightforward dismantling and removal, at an expense of under two percent of the cost of the plant. Immediate dismantling under remote control has been shown feasible in an experiment performed in the Nevada desert, but for a 1,000-MW unit it could cost as much as $300 million if performed immediately or $150 million if delayed for 30-40 years.
PRACTICALITY AND MORALITY
If nuclear waste disposal is so safe, why can't conventional waste disposal be made as safe or safer? Because the sheer quantity is overwhelming. As in all other aspects of nuclear power, its superior safety is ultimately due to the concentration of danger in small volumes that can easily be guarded and protected by multilayered safety systems. Such systems are simply not thinkable for containing other dangers.
Transportation is a good example of this principle. Hardly a week goes by without an evacuation somewhere because a train carrying chlorine or ammonia or some other toxin has derailed. The amounts produced (and presumably transported) in the US every year are 16 million tons of ammonia, 9 million tons of chlorine gas, 32 million tons of sulfuric acid—the list could go on for pages. These vast quantities simply cannot be protected from leakage, derailment, fire, and other hazards.
But nuclear wastes are so minute in quantity that it is eminently feasible to design containers that will withstand crashing into concrete walls at 60 miles per hour, dropping onto spikes from a height of 30 feet, and surviving prolonged periods in the open flames of a fire. Theoretically, the same could be done for chlorine gas. But for 9 million tons of it?
"The best practicable technology" is a phrase included in most environmental regulations. However much people may object to this fact, the evidence shows that the best practicable technology for the wastes arising in the generation of electricity is to generate it by nuclear energy in the first place. The present method of disposing of those wastes kills some 39,000 Americans a year. The victims of nuclear waste disposal, if any, will be quite negligible compared with that number. How long can the opponents of nuclear power continue to conceal this comparison?
It seems likely that nuclear power will ultimately have to replace our present methods of generating electricity. The reason, which has little enough to do with morality, is evident at a glance from a figure drawn up by the London Economist to depict the various quantities of energy producible from estimated reserves of resources (see figure at left). Opposition to nuclear power can hold it back for a year, two years, perhaps even a decade or two. But for every 60 minutes spent in speaking against nuclear power, four more American victims of the present method of electricity-linked waste disposal meet an untimely death.
Petr Beckmann is a professor of electrical engineering at the University of Colorado and the author of a number of books and scientific papers. Originally working in electromagnetics and probability theory, he became Interested in questions of energy and now publishes the monthly newsletter Access to Energy. This article is adapted and updated from his booklet The Non-Problem of Nuclear Wastes. Copyright © 1979 by Golem Press.
*The waste quantities cited assume a capacity factor of 75 percent, coal with 18 percent ash factor (Midwestern bituminous), and a conversion rate of 2,500 kwh per ton of coal.
IS IT ALL WASTE?
Most of nuclear waste is useless, and its most important aspect is replacement of far more voluminous and far more damaging waste from alternative methods of power generation. Yet some of it is useful now, and some more may be useful in the future (making a case for storing nuclear wastes retrievably).
Some fission products can be used as tracers. Since the chemical properties of an isotope do not depend on whether it is radioactive or stable, the former variety can show where a chemical is located. (How is iron distributed over the cross-section of a tomato, for example?)
The same idea of chemical equivalence has been tried to produce chemicals that will seek out cancerous cells and kill them with radioactivity. (Many people frightened by all things nuclear have pretty much forgotten that radioactivity is used to cure cancer.) In preliminary experiments cited by Edward Teller (Energy from Heaven and Earth), 70 percent of the rats so treated recovered from breast tumors, whereas the untreated animals died.
Food decays via the action of bacteria, which can be killed by radioactivity without affecting the food. Grain, fruit, and vegetables can thus be preserved without the use of chemicals. (Only South Africa has so far been courageous enough to use this method commercially.)
Currently, water supplies are disinfected by chlorination, but this produces chlorinated organics that are suspected of being carcinogenic. Gamma rays and their secondary products are capable of breaking up these compounds, as well as destroying other chemical pollutants such as phenols, cyanides, and benzo-apyrene.
The sludge produced by sewage treatment plants can also be irradiated in order to destroy the disease-spreading bacteria breeding in it; it does not itself become radioactive in the process. This would not only sterilize the sludge but make it useful as a fertilizer and even as animal fodder.
Nuclear wastes could also provide heat to power electric generators in unmanned installations. In the Arctic and Antarctic, energy for meteorological stations is in fact being provided by strontium-90 recovered from spent fuel rods and packaged for such use.
A particularly intriguing point is the recovery of rare metals essential for some alloys and other applications but now available only from Zimbabwe and South Africa (sources that are considered politically unreliable enough to spark the current concern about strategic metals). In particular, rhodium, palladium, and ruthenium, each of which is more precious than gold, are present in the fission products, where latter-day alchemy has produced them from cheap uranium ore. It is not yet economic to extract these elements from nuclear wastes, but one day it evidently will be.
This article originally appeared in print under the headline "Containing Nuclear Waste."


Show Comments (0)