'Editing' Life With Gene Drives is a Great Way to Play God
Researchers develop a powerful new tool to manage wild ecosystems.
Woul

dn't it be great if scientists could genetically engineer mosquitoes to be immune to the malaria parasite, thus protecting people from that disease? How about restoring the effectiveness of a pesticide by eliminating resistance genes in weeds and insect pests? Or altering genomes to eradicate a pesky invasive species? These are exactly the sorts of things that a brand new biotechnological tool could do, and it's got some people worried.
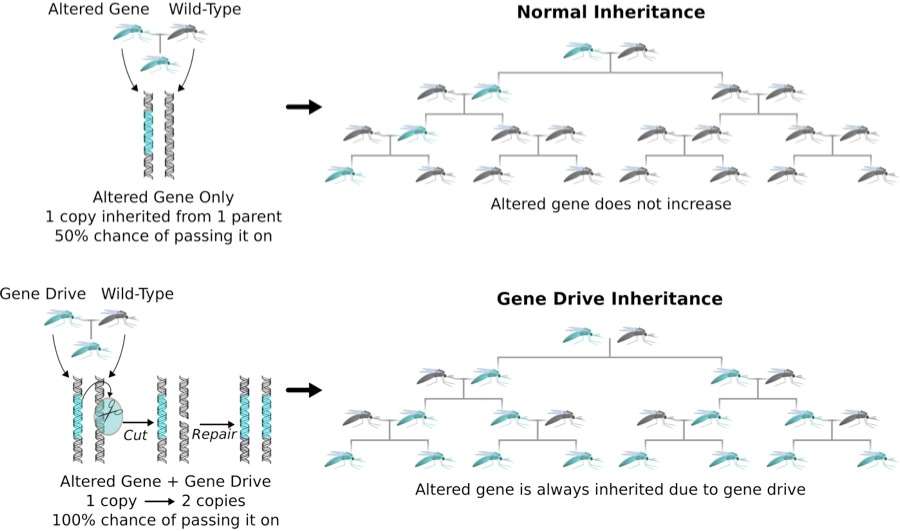
In a July article for the journal eLIFE, a team of researchers led by the Harvard biotechnologist Kevin Esvelt outlines a system that uses the new CRISPR gene editing technique to alter the genomes of wild populations of plants and animals. CRISPR is based on a bacterial protein that can identify and cut any desired segment of DNA in an organism's genome. Appropriately configured and guided, it can replace any gene with a newly engineered version. Esvelt and company want to use CRISPR to construct "gene drives" that can quickly spread beneficial engineered genes through sexually reproducing populations. A gene drive works by making sure that both copies of a targeted natural gene are replaced with the engineered version.
For example, researchers could take a gene drive specifying an anti-malarial protein and insert it into mosquitoes in the laboratory. They would then release the insects to breed with wild mosquitoes. Ordinarily, the progeny would get one copy of a gene from each parent, but in this case, the gene drive would excise and replace the natural copy from the wild parent with the anti-malarial version, thus guaranteeing that it would get passed along when the next generation of mosquitoes breeds. Eventually, essentially all of the mosquitoes in the targeted species would carry the engineered version.
Another proposal would get rid of invasive species by creating a suppression gene drive that biases the production of sperm containing Y chromosomes, so that only males are born. The spread of the Y-drive would result in a population crash of the targeted species.
And then there's a potential solution to a big agricultural problem: weeds and pests that develop resistance to herbicides and pesticides over time. Researchers could create sensitizing gene drives that would replace resistant alleles with their vulnerable ancestral equivalents and thus restore the effectiveness of the herbicides and pesticides.
Naturally the development of a technology this powerful freaks some people out. The Hastings Center bioethicist Gregory E. Kaebnick told The Boston Globe that he "would be opposed to playing around with this technology unless there are very significant benefits." Todd Kuiken, a biosecurity analyst at the Woodrow Wilson International Center for Scholars, added that scientists "need to understand there may be technologies that, once you debate them, the public may decide we don't want to move forward on this."
Gene drives could go wrong, as Esvelt and his colleagues acknowledge. Engineered drives might spread to non-targeted species through interbreeding. Suppression drives that aim to crash a population of an invasive species might spread back to that species' natural habitat. Bad guys might try to use gene drives to damage crops and livestock. And then there's the possibility that gene drives might be used to alter the genetics of human beings.
Gene drives would be released into the wild, which is the equivalent of an open access commons. The Harvard biologist George Church, a co-author of the eLIFE article, writes in Scientific American, "Because we are all affected by the state of our ecosystems, public oversight of technologies capable of ecological management will be essential." Owing to these concerns, Esvelt and his colleagues make some suggestions about how to regulate gene drives in a companion article for the journal Science.
First, they describe some technical fixes. Before any gene drive is released into the wild researchers should create a reversal drive that can restore the original phenotype of a targeted species. Suppression drives should be released only after researchers have developed a corresponding immunizing drive that would prevent a specific unwanted drive from being able to spread. Such precautionary measures would enable researchers to swiftly counteract the effects of an accidental release.
One technically sweet proposal for how suppression and immunizing drives could be used together is to let loose a suppression drive among invasive rat populations on islands while simultaneously releasing an immunizing drive in Europe and Asia to keep the suppression drive from spreading among native rat populations. Of course, rats with immunizing drive might eventually re-invade habitats where they are not wanted, so researchers would over time have to develop different suppression and immunizing drives to keep up.
Gene drives would first be tested among captive populations of targeted organisms confined to laboratories or located in geographically isolated areas far from native wild populations. The presence and prevalence of gene drives would need to be monitored through periodic testing of genomic samples taken from the wild.
The researchers discount the possibility that villains could successfully use gene drives to attack a country's crops and livestock. In developed countries, they argue, gene drives would be quickly detected by seed and livestock companies, and carrier organisms would be purged before they could breed extensively. In a poor country where farmers still save and replant seed and breed their own livestock, agriculture might be more vulnerable. But even there, if something appeared wonky, modern genome testing could quickly identify such an attack and countermeasures could be taken.
Esvelt and his colleagues downplay the possibility that gene drives could be used to change human genetics. "Gene drives will be ineffective in altering human populations because of our long generation times," they claim. "Furthermore, whole-genome sequencing in medical diagnostics could be used to detect the presence of drives."
The technology isn't ready to be deployed yet, but we're getting close. As George Church told The New York Times, "In a year or two, we could be doing field trials if there was a general consensus this was a good idea." The usual luddites will strive mightily to scare policy makers into banning gene drives. But with the proper safeguards, the benefits clearly outweigh the possible downsides. To prove that, let's go after malaria first.


Show Comments (20)