I'll Show You My Genome. Will You Show Me Yours?
Our science correspondent reveals his genetic code. Soon you will too.
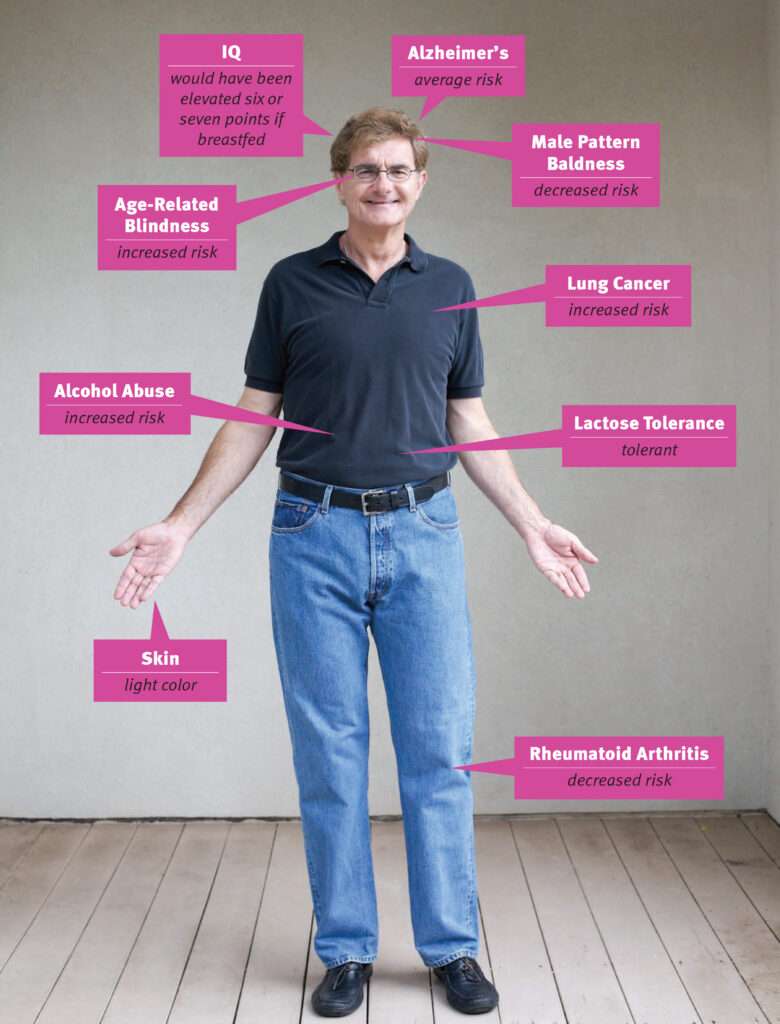
Michael Cariaso, developer of the human genetics wiki SNPedia and the online gene analysis tool Promethease, has helped thousands of people unlock the secrets of their own genetic code. But when it comes to making his own gene screening tests publicly available for all the world to see, Cariaso prefers to hold the key close to his vest, worrying that such transparency might lead to personal embarrassment or discrimination by insurance companies or future employers. "Someone later might discover," he says, "that I have genes for a short penis and low intelligence."
Cariaso is certainly a smart guy, and he is hardly alone in his general concerns. (With regard to his genitalia, as the philosopher Wittgenstein said, "Whereof one cannot speak, thereof one must be silent.") But he's wrong. Fears about the loss of genetic privacy are greatly exaggerated. We are fast approaching an era in which genetic information is no longer exclusive or medicalized. Instead, as screening costs plummet and our knowledge about genetics expands, virtually everyone will soon be able to have their genotypes at their fingertips. Knowing and sharing that information will enhance, not jeopardize, our sense of ourselves, change the way we consume medicine and plan for the future, and influence how we relate to each other.
That's why I've decided to post my genotype screening information online. You can read all about me at snpedia.com/index.php/User:Ronald_Bailey. As a service to future consumers and as a guide to the world we will all soon be living in, here are my answers to the most common questions about and objections to genetic testing.
How does genetic screening work?
Right now the cheapest, simplest way for a consumer to get some preliminary insight into his or her genetic makeup is to pay a few hundred bucks for the services of a gene screening company, such as 23andMe, deCODEme, Navigenics, or Pathway Genomics. Unlike colonoscopies or even ordinary blood tests, a gene screen isn't gross, scary, or inconvenient. Simply spit into a test tube, send it off, and a few weeks later you get a read-out of up to 1 million single-nucleotide polymorphisms (SNPs) from your genome. SNPs are variations in an individual's genetic code that are useful in understanding differences among people and for identifying some disease risks.
In May 2010 one company, Pathway Genomics, even arranged to offer its screening test over the counter at Walgreens drugstores. Unfortunately, a hyper-cautious Food and Drug Administration (FDA) sent a letter to Pathway Genomics asserting that the test was a regulated medical device, prompting Walgreens to postpone selling the service for now. In June the FDA sent a letter to other gene screening companies, to test-chip maker Illumina, and to the whole-genome sequencing company Knome, ordering them all to show why their tests should be exempt from the agency's premarket clearance regime for regulated medical devices. In his letter, the FDA's Alberto Gutierrez expressed worry that "consumers may make medical decisions in reliance on this information." Well, yes; that's the whole point.
People already are making those same decisions, but with much less information. Vague stories about Aunt Sally's breast cancer prompt a mammogram; an uncle's heart attack leads to some half-hearted jogging. People make health decisions all the time. The FDA's aggressive regulation of direct-to-consumer gene testing does little more than keep information away from decision makers.
Despite these regulatory travails, there are at least nine companies in the U.S. that will go beyond checking for gene variants and will soon offer to decode all 3 billion DNA base-pairs in a person's whole genome. This is astonishingly rapid progress from very expensive pure science to relatively cheap commercialization. The first complete human genome was sequenced back in 2000, a government project that cost around $3 billion. In November 2009, the privately held Complete Genomics sequenced a whole human genome for just $1,700. One company, Pacific Biosciences, claims that by 2013 it will be able to map a consumer's genome in 15 minutes for less than $1,000. Many of these companies are competing for the $10 million Archon Genomics X Prize funded by the Canadian geologist and diamond mine entrepreneur Stewart Blusson and his wife Marilyn Blusson, which will be awarded to the first group to build a device that accurately sequences 100 human genomes in 10 days for less than $10,000 per genome.
I doubled up on genetic testing, receiving reports from two different companies, 23andMe and Pathway Genomics. I signed up for an early 23andMe test at $1,000 and a later Pathway Genomics test for $399. In both cases, about six weeks after I sent off my spit, I received an email message telling me my test results were available. I got 23andMe's results first, and I logged onto its website with the kind of happy anticipation one feels opening birthday presents.
What do results look like?
The good news: I have low odds of suffering from male pattern baldness, and my chance of getting rheumatoid arthritis is less than 1 in 100, compared to the average risk of 2.4 per 100 people.
Unfortunately, I carry two gene variants that increase my risk of age-related macular degeneration and one variant that reduces the risk, which means that combined my risk of going blind is 9.5 out 100, compared to the typical risk of 7 out of 100. It is just as well that I have no plans to become a competitive short-distance runner, since I do not have the gene variants for fast twitch muscles often found in world-class sprinters. I also have gene variants suggesting that being breastfed likely would have raised my IQ by six or seven points.
The tests revealed there's no truth to the family legend that we're related to a Cherokee princess. 23andMe's ancestor screening tests suggest that it would be hard for someone to be more genetically European than I am. According to my mitochondrial DNA, my maternal line descends from Haplogroup U5, which arose among early Homo sapiens sapiens colonizers of Europe around 40,000 years ago. According to my Y chromosome, my paternal line appears to hail from Ireland. The results from Pathway Genomics later confirmed this genealogy.
In the course of making information understandable to users, both 23andMe and Pathway Genomics generally cite research that has been strongly confirmed by the peer-reviewed literature. But if you're hankering for more detailed and speculative insights into your genetic information, you can run the raw data through Promethease, a trait analysis tool that links test results to research reports compiled at the wiki-style SNPedia. As is often the case with crowd-sourced information, SNPedia is comprehensive but messy. When I compare the reports from 23andMe and Pathway Genomics with the results I got at Promethease, however, all three pretty much agree.
Promethease tells me I have the complement of alleles strongly suggesting that I am male. Very reassuring. It also agrees with 23andMe that I have some alleles indicating a low probability of being bald. Check. An allele for light skin color. Check. A set of alleles showing I can digest milk as an adult. Check again. I was also happy to learn that my risk of ovarian cancer is likely one-third lower than average.
Skimming through Promethease, I also find I have gene variants that raise my IQ by seven points. In this regard I am not particularly special, since 30 percent of people have the variant that confers four extra IQ points and 47 percent share the variant that adds three. People who don't have these specific variants may well have versions of genes that boost their IQs in other ways.
Is the information useful?
Most people don't need genetic testing to find out whether they are well-thatched white males who can digest milk. Confirming the obvious is gratifying, but genetic testing is supposed to offer access to the unseen future.
Since gene hunters generally try to figure out how heredity correlates with disease, the gene testing companies and Promethease produced a lot of information about how my genes might contribute to illness. In my case, it looks like it was a good idea to quit my three-pack-a-day cigarette habit 23 years ago. According to Promethease, I have several gene variants that significantly boost my chances of lung cancer, accelerated lung decline, and congestive obstructive pulmonary disease. Pathway Genomics confirms this result, noting that "your genetic profile suggests that you may be vulnerable to lung cancer." I also have a variant that correlates with staying addicted if one becomes a smoker, which makes me wonder about a possible willpower gene hiding somewhere.
Cardiovascular disease risk is particularly interesting to me since my father died of a heart attack at age 70 and my mother, who suffered several heart ailments, died of a stroke at the same age. Heart disease is the leading cause of death in the United States: According to the American Heart Association, 830,000 Americans died of cardiovascular disease in 2006, some 425,000 of them from coronary artery disease. So it's not surprising that I, like many people, have genes conferring some risk of heart disease.
Promethease finds that I have an SNP that increases my chances of coronary artery disease by 50 percent above average. Pathway Genomics measures 12 different markers for coronary artery disease and 11 gene variants associated with the risk of heart attack. The results suggest I am somewhat more susceptible than average to both. 23andMe tests for only one variant, which indicates that my risk of heart attack is slightly below average, at 20.9 out of 100 Caucasian males, whereas the average is 21.2 out 100. All three platforms find that I am at greater risk than average for experiencing atrial fibrillation, a heartbeat characterized by a fast irregular rhythm.
The differences in reported results between the companies arise not from inaccuracies but from their selection of studies to include in their analyses and their interpretation of the research. In any case, a 64-slice CT heart scan a few years ago showed that I had no sign of significant coronary blockages. Also, my total cholesterol level is 167 milligrams per deciliter, well below the 200 milligrams per deciliter threshold that increases heart disease risk.
What other genetic flaws did Promethease suggest? I have one copy of a gene variant that increases my relative risk of type 2 diabetes by 10 percent; I also have two copies of an allele that increases my chances of type 1 diabetes to 20 times the average. That sounds bad, but recent tests show that my blood glucose levels are well within the normal range, indicating that I don't have diabetes.
My diabetes results illustrate an important concept: There is no single gene for such common ailments as heart disease, diabetes, or cancer. Instead, hundreds of genes combine with influences from the environment to either increase or reduce risks. As the saying goes, "Genes load the gun; the environment pulls the trigger."
The more useful information that gene scanning can provide today is how we will likely react to various pharmaceuticals. "Drug response can be predicted accurately for more than a dozen drugs," noted National Institutes of Health Director Francis Collins in the April 2010 issue of Nature. For example, 23andMe, Pathway Genomics, and Promethease all suggest that I would respond strongly to the blood thinner warfarin, which would mean that, should I ever need it, my physician should probably aim to stabilize me at a lower dose than average. By contrast, all three sources report that I should respond typically to the blood-thinning drug Plavix. In March the FDA updated Plavix's label to inform physicians that there is now a genetic test to determine how patients will respond to it. Given my genetic risks for heart disease, Pathway Genomics reports the good news that people with my "genetic markers receive significantly greater benefit from intensive statin therapy (such as Lipitor) than people who do not have these markers."
But in general, the results of current tests are probabilistic calculations based on a selection of low-risk susceptibility alleles. The right way to think about the current direct-to-consumer genotype screening tests is that they are a preliminary technology. They offer supplementary, not dispositive, information about various health risks. The tests are not perfect, but they are the beginning of the process through which consumers, physicians, and purveyors will learn how to better interpret and use genetic information over time. The only real response to many disease risks right now remains that hoary but correct advice to eat your vegetables, lose weight, exercise more, and not smoke.
What about Alzheimer's?
Alzheimer's disease envelops us in a fog of forgetting, gradually stealing our memories, minds, and identities. It robs us of our dignity, leaving us a helpless burden on our families. The prospect of Alzheimer's disease is so frightening that two prominent researchers who have had their genomes scanned—James Watson, co-discoverer of DNA's structure, and Steven Pinker, a cognitive psychologist at Harvard—declined to learn what their gene tests have to say about their risk of it. Specifically, they didn't want to know if they carry copies of the APOE4 allele, which boosts the odds that a person will eventually get Alzheimer's to as much as 20 times the average. (More happily, recent research suggests that people carrying APOE4 alleles have better memories in their youth than those who carry the APOE3 variant.)
Unlike Watson and Pinker, I do want to know. Not all gene screening companies include APOE4 testing, but Pathway Genomics does. The good news is that my failing memory is not due to APOE4; I have inherited two copies of the more common APOE3 variant, which suggests that my lifetime risk of Alzheimer's disease is average. Of course, there are other gene combinations that can increase or decrease my risk.
Back in 1999, as APOE testing was becoming more widely available, a panel of bioethicists convened at Stanford University concluded that Alzheimer's testing was inappropriate for most individuals. The bioethicists were concerned about the "impact of knowing one's own genetic susceptibility to an incurable disease." In particular, they were afraid that consumers would "make significant life decisions based on a misunderstanding of risk estimates." They also feared that insurance companies might use such test results to discriminate against people in issuing and setting rates for health and life coverage.
Ten years later, researchers led by the Boston University physician Robert Green reported the results of a study designed to find out how people actually would react to test results suggesting their risk of Alzheimer's disease was considerably higher than average. The results are reassuring: People can handle the truth. The researchers gave APOE tests to 162 asymptomatic adult volunteers who each had a parent with Alzheimer's disease. They randomly assigned the participants to either a group which was told their results or another that was not. The researchers monitored the participants for symptoms of anxiety and depression over the course of a year.
Not surprisingly, people who were told they tested negative for APOE4 felt relief. But those who tested positive experienced only transient distress, undergoing no more anxiety or depression than people in the nondisclosure group. "The disclosure of APOE genotyping results to adult children of patients with Alzheimer's disease did not result in significant short-term psychological risks," the study concluded. Another study reported that participants who learned that they carried the APOE4 allele were more likely to buy long-term care insurance.
What if other people find out my genetic secrets?
The chief reason that most people worry about genetic privacy is potential discrimination by insurers and employers. So what happens if someone receives my résumé and decides to pop over to Promethease to take a look at my gene scan information?
Among other things, they would find that I have a gene variant that some studies suggest can increase my risk of substance abuse (of both alcohol and "street" drugs) fourfold. To make matters worse, one of the gene variants that increases my risk of lung cancer is also associated with a higher risk of alcoholism. Then again, I don't have a gene variant associated with strong alcohol cravings in some drinkers.
I confess that I enjoy a shot of single malt (OK, usually more than one) from time to time, and that dining out with me usually involves sharing more than one bottle of wine. We'll leave my street drug history back in my 20s, when it occurred.
Might an employer decide, looking at my profile, that he doesn't want to hire a possible drunk? For now, the Genetic Information Non-Discrimination Act (GINA), passed in 2008, prohibits employers from asking job applicants for genetic information or using it in making employment decisions. The federal government's Equal Employment Opportunity Commission has ruled that the "acquisition [of genetic information] through commercially and publicly available documents like newspapers is permitted, as long as the employer is not searching those sources with the intent of finding genetic information." So reading this article is OK, but seeking out data on Promethease is evidently prohibited.
At any rate, I would have no concerns about disclosing my genetic information even without GINA in the picture. The law is policy overkill, and it will turn out to be largely superfluous once most people realize that genetic information is not somehow special, toxic, or occult.
The biggest concern may be not the genetic analysis available now but what we figure out later. What if future research turns up genes associated with criminal behavior, for instance? I have two copies of the "warrior" version of the catechol-O-methyltransferase gene, which correlates with higher functioning in a crisis, possibly because it confers some protection against anxiety and pain susceptibility. The alternate "worrier" version of the same gene is associated with better memory and more focused attention, but individuals carrying it may crack under pressure. In addition, research published in the April 2010 issue of Neurology suggests that the warrior gene helps prevent cognitive decline as people age. Then again, some studies associate it with higher levels of aggression and greater risk of schizophrenia.
For the record, I haven't been in a physical fight since the eighth grade and have not been arrested so far. And late-onset schizophrenia is quite rare. But right now, an employer naively using the results of my, or anyone else's, genetic tests to make hiring and firing decisions is likely to be misled by the very preliminary information that gene screening currently makes available. It would be like deciding to pass over first baseman Albert Pujols if his gene scan indicated that he might have a slightly higher risk of alcoholism, or turning away physicist Richard Feynman because he had an SNP combination suggesting a tendency toward aggression.
After all, genes are not destiny, especially genes for relatively common complex traits and diseases. Even while having my share of hangovers, I have managed to support myself and more or less satisfy my employers since the age of 18.
What about kids?
In 2009 I asked Harvey Fineberg, the director of the Institute of Medicine, the health arm of the National Academy of Sciences, if there were any good reasons not to reveal your genetic information to the public. Fineberg replied that doing so might worry your children or embarrass them in front of their peers, if your genes hint at, say, a heightened risk of substance abuse or some medical debility.
In the age of cheap, easy genetic testing, checking your kid for deleterious genetic conditions that might be ameliorated by current treatments is the only responsible thing to do. But what about genetic testing for conditions that manifest only in adulthood, or for which there are no treatments? A 2009 survey in the journal Pediatrics found that "one third of parents are interested in predictive genetic testing for their children, even for disorders with no treatment." One third were unsure, and one third said that they had no interest in it.
The Genetic Information Non-Discrimination Act does not ban parents from having their children's DNA tested. The National Society of Genetic Counselors cautiously advises parents to include their children in decisions to test for adult-onset diseases and to think seriously about whether the decision to test should be reserved for the child to make upon reaching adulthood. Pathway Genomics currently will not test people who are under 18. 23andMe leaves the decision to parents, who can submit samples from children younger than 18.
But I think they're all worried about the wrong thing. Some time before the end of this decade, kids are going to be running gene scans and maybe even whole genome sequencing experiments in their ninth-grade biology classes, just the way some of us did blood typing experiments back in the mid-20th century. Then they are going to share that information with their friends on whatever social media follow Facebook and Twitter, and they'll do it without parental consent. Nerdy high school sweethearts might swap DNA profiles and run them through computer programs designed to predict what their potential children might look like. In the process, of course, they will also be sharing information about their parents' genes.
We live in a society of increasingly radical self-disclosure and transparency, and genetic information will not be immune to this trend. Many genetic testing customers are already sharing information among themselves. The 23andMe customer website hosts numerous groups of customers organized around shared ancestry or disease concerns. Many people have the impulse to share more, not less, when they get bad news. Today practically any disease you can imagine has multiple online sites where patients and caregivers can commiserate, exchange information, and advocate research. "Take bipolar disorder," says 23andMe co-founder Linda Avey. "There's been a complete change. People used to hide it away—it was a real embarrassment. Now people blog about their bipolar disorder. It's fine to be open about it. That's the same thing we see with genetic data. People want to share genetic information."
I posted a general question at the 23andMe community forum about whether anyone had had any negative or positive experiences as a result of revealing their genetic information to someone. One 23andMe customer said she had offered to pay for tests for her siblings, who declined because of privacy concerns. But several people mentioned what they considered to be positive experiences. One 23andMe customer said she told her knee surgeon she had a fourfold higher risk of blood clotting in her legs. "He prescribed an injectable anti-clotting medication instead of the standard post-operative care," she wrote. "There's no way to tell if that actually affected the outcome, but I'd classify my physician's response as very receptive." Another customer said that when she had her annual physical, she gave her physician her 23andMe results regarding likely response to anti-cholesterol statin drugs. "I was on the tipping point for using statins," she wrote. "My 23andme results, along with my blood work, helped my doctor and me to decide to start an anti-cholesterol drug."
What if the government uses my genes against me?
This is the most worrying aspect of our genomic future. Right now the U.S. National DNA Index System, run by the Federal Bureau of Investigation, contains nearly 8.5 million genetic profiles, some of them of convicted criminals and others of people who have merely been arrested. These profiles are genetic "fingerprints" consisting of 13 specific segments of DNA that contain no genes. The data can be used only to identify criminal suspects, offering no information about a person's medical conditions.
The American Civil Liberties Union opposes collecting genetic information from anyone who has not been convicted, on the grounds that under the law people are regarded as innocent until proven guilty. It's a valid objection, but it is hard to see how this view can prevail, since the FBI already maintains a database containing more than 250 million sets of fingerprint records, both criminal and civil. In a March New York Times op-ed piece, Michael Seringhaus, a Yale law student, argued on fairness grounds for establishing a national DNA database containing the genetic profiles of every U.S. resident. Who needs a national ID card if every cop has a fast DNA reader and wireless electronic link to the comprehensive national DNA database? We are likely to hear more such proposals.
In another worrying development, Israeli researchers last year reported that they were able to manufacture fake DNA samples using government data. Law enforcement officials or others could salt a crime scene with fake DNA as a way to frame an innocent person. Interestingly, the privacy protections of the Health Insurance Portability and Accountability Act specifically forbid law enforcement agencies to obtain genetic information from patient records held by hospitals and physicians without a court order. But as predictive genetic information is incorporated into the new U.S. national health care files, government agencies probably will succumb to the temptation to use it when making decisions about how to allocate medical and educational resources.
As government DNA databases grow, concerns about state abuse of genetic information deserve serious consideration and debate. But individual discretionary disclosure isn't central to this debate. Either we will pass strong legislation preventing the government from getting access to this information or—more likely, alas —the authorities will be able to build their database anyway, regardless of whether or not we choose to disclose any genetic information voluntarily.
Will people with risky genes be able to get insurance?
Recall one of the results from the Alzheimer's study: Many carriers of the deleterious APOE4 allele decided to buy long-term care insurance. This is an example of what insurers call adverse selection, the tendency for insurance to be purchased chiefly by those who are most likely to need it, thus raising its cost and reducing its benefits. As sicker people pile into an insurance pool, the price goes up and the healthier flee, producing an insurance death spiral of ever-higher premiums and ever-fewer buyers. "If everybody knows if they are going to be sick or healthy, only those expecting to be sick will buy insurance," says Thomas Wildsmith, a director at the American Academy of Actuaries.
But how are insurers using genetic information so far? Wildsmith notes that even before the Genetic Information Non-Discrimination Act, "insurers were not asking anyone to take genetic tests." He adds, "The truth is that most insurers are not that sophisticated about using genetic information."
Genetic information is not all that relevant in the current health insurance market. First, most people get their insurance through group plans offered by their employers, so the average risk of the groups is what matters. And what about the market for individual health insurance policies? "Take the case of a 25-year-old seeking health insurance," suggests Wildsmith. "His dad had a coronary at age 50. Who cares? It's not important because he'll drop the policy by age 50."
Some states have allowed insurers to take a person's body mass index, cholesterol levels, blood pressure, and other factors into account when underwriting individual health insurance policies. But all this is now largely moot, since the health care legislation passed last spring by Congress explicitly forbids insurers from taking into account pre-existing conditions—including the results of genetic testing—when setting rates. Wildsmith suggests that the only sure way to avoid an adverse selection spiral now is to "make the healthy people buy insurance too." Which is exactly what Congress did when it mandated that every American must buy health insurance.
But what about the effects of truly predictive genetic testing on markets for long-term care, disability, and life insurance? People tend to buy and keep such policies for decades. As would-be policyholders obtain more information about their genetic risks, many likely will seek to purchase such policies. If insurers are kept in the dark about risks that their customers know, they will be at a disadvantage in setting appropriate rates. A 1999 article in the North American Actuarial Journal outlined the three choices facing insurers and customers: Buyers could choose not to take genetic tests, and the result would be higher risk premiums for their policies. Buyers could take the tests but refuse to disclose the information to insurers. Again, the result would be higher risk premiums. Or buyers could disclose their test results to insurers, which would allow them to charge an actuarially fair premium. Of course, if tests suggested that a buyer has relatively high disease or mortality risks, the result would be higher premiums.
In 2008 Karen Pollitz, director of Georgetown University's Health Policy Institute, wrote in the journal Managed Care, "Much of life's uncertainty about health will become much more known to us, and since insurance is all about protecting people from the unknown, that will be a profound change." So profound that Pollitz thinks it could ultimately make the insurance industry obsolete. By contrast, Fei Yu, an actuarial researcher at Heriot-Watt University in Scotland, argues that advances in health care and the general trend toward mortality improvement will overwhelm genetic risks. In other words, our genes will exercise less and less power over our health destinies as our medical knowledge and technologies are perfected.
The scope of genome-informed medicine is vast. Alan Guttmacher, former acting director of the National Human Genome Research Institute, noted at a 2009 Institute of Medicine meeting that all current drugs, including over-the-counter, prescription, and street drugs, target only 500 or so of our genes. That's maybe 2.5 percent of the entire human genome. It may be that only half of our genes are druggable, but that leaves huge scope for new disease treatments targeted at specific genes.
Can people be trusted with their own genetic information?
The field of genetic testing has already attracted some charlatans. In 2006, for example, the Government Accountability Office (GAO) investigated the claims being made by a number of "nutrigenetic testing" companies that promised to give customers dietary advice tailored to their genetic proclivities and sell them "personalized" supplements. According to the GAO report, "The results from all the tests GAO purchased mis-lead consumers by making predictions that are medically unproven and so ambiguous that they do not provide meaningful information to consumers."
People and companies peddling fraudulent information of any sort should be prosecuted. But government's criticism extends beyond such chicanery. In July the GAO reported on another sting of genetic testing companies, including 23andMe and Pathway Genomics. The GAO declared that the results of genetic screening tests were "misleading and of little or no practical use." The chief basis for this conclusion was that the GAO's investigators sent the same genetic samples from five people to all four screening companies and were surprised that the results were not identical.
The results differed, however, largely because each of the screening companies selects the markers it considers most relevant and the studies it deems most illuminating. That's why 23andMe and Pathway Genomics disagreed about my risk for heart attack. But is the information offered by the main genotype screening companies accurate and valid?
Yes, it is. "I ran an analysis on personal genome results obtained from 23andMe and DeCODE for me," says the Princeton biologist Lee Silver. "There were about 300,000 data points that overlapped between the two tests. There was not a single data point (among 300,000) that was scored positive in one test and negative in the other."
Nevertheless, genetic information is complicated; many customers are likely to misunderstand some of it. For some bioethicists, the solution is to keep consumers ignorant by banning or at least strictly regulating access to genetic tests. Hank Greely, director of Stanford's Center for Law and the Biosciences, told The Washington Post in May that offering Pathway Genomics tests on drugstore shelves is "reckless." While "information is powerful," he said, "misunderstood information can be powerfully bad."
But how big is that risk? A 2009 study led by Colleen McBride of the National Human Genome Research Institute evaluated the responses of patients who accepted an offer for genetic susceptibility testing for eight different conditions. The results were reassuring: "We found no evidence that those who considered or sought testing were inclined to overestimate the contributions of genetics to common health conditions or to underestimate behavioral risk factors." There was a bonus: Many people whose tests suggested they were at higher risk for some diseases were motivated to engage in healthier activities, such as losing weight and exercising more.
People can misunderstand new information. But the way that consumers learn how to use any new product is by trying it out. If the first purchasers of the new Pathway Genomics tests find them confusing or not very useful, they will tell their friends and neighbors, and Walgreens will find some new vitamin mixture or cosmetic to take up that shelf space. Instead of trying to slow down social learning about genomics, we should let companies and consumers interact so they both can learn how better to explain and understand the information such testing provides.
What's next?
Gene screening may not be for everybody right now, but I am confident most people will find it useful and even entertaining sooner rather than later. Before the end of this decade, if federal regulators stay out of the way, advances in personal genomics will bring enormous health benefits to the public. More medications will be targeted to the specific genetic makeup of individual patients, improving the chances of a cure while minimizing debilitating side effects.
Already cancer treatments are being honed using genetic tests of individual patients' tumors. For example, patients who score low on the Oncotype DX genetic test for breast cancer recurrence can avoid the physically brutal consequences of traditional post-surgical chemotherapy. Researchers are working on wide-spectrum tests that could identify the genetic signatures of diseases in patients before they are manifest. Other tests will warn prospective parents of possible deleterious gene combinations in their future progeny. The ongoing exponential growth in our genetic knowledge may even uncover ways to retard the aging process.
We are in the Apple II era of genetic testing. It would have been silly to ban the Apple II just because it was not as easy to use or immediately comprehensible as the MacBook Air. Standardization of test results will come as information accumulates about the interaction between genetic variants and environmental influences. The current tests function as practice runs for curious consumers. As one of those early adopters, I don't want or need federal regulators to protect me from my own test results. There are things I want to keep private, but my genes aren't one of them, no matter what they may reveal about my intelligence and genitals.
Ronald Bailey (rbailey@reason.com) is reason's science correspondent.


Show Comments (48)