Human Challenge Trials for Coronavirus Vaccines Are Ethical and Should Be Initiated Quickly
Volunteers would earn cash for the risks they take on our behalf.
It's time to recruit some healthy young people, inject them with various experimental coronavirus vaccines, and then expose them to the virus to see if any of them work. The health of the volunteers would be monitored carefully, and they would be given access to the best available care should they need it. Besides the satisfaction of perhaps playing a role in saving hundreds of thousands of lives and hastening the end of the lockdowns, the volunteers could earn some cash for their troubles as well.
An article today in Science by the Northwestern University bioethicist Seema Shah and her colleagues lays out an ethical framework for initiating such controlled human infection studies (CHIs) to speed up the process of identifying effective vaccines and treatments for the virus that causes COVID-19. Such studies, they write,
could have high social value in several ways. For example, they could help prioritize among the almost 100 investigational vaccines and over 100 experimental treatments for COVID-19 currently in development. CHIs could help identify the most promising agents, which would inform the design of larger trials, guide decisions to scale up manufacturing early, and thereby accelerate product development and implementation. If they saved even a few months of vaccine development, [coronavirus] CHIs would contribute to faster control of the pandemic and reduce the need for, and associated costs of, physical distancing measures, providing substantial benefits for much of the world's population (including the most vulnerable).
Not least among the "substantial benefits" that CHIs could offer is a faster restart and recovery of the world's economies.
So those are the benefits. What about the risks to the volunteers? The ethicists observe that some research estimates that "healthy adults aged 20 to 29 have a 0.03% risk of death and a 1.1% risk of hospitalizations" from COVID-19. Thus, they suggest, the risks young CHI volunteers would run are similar to risks people typically take in other research and in some aspects of every day life:
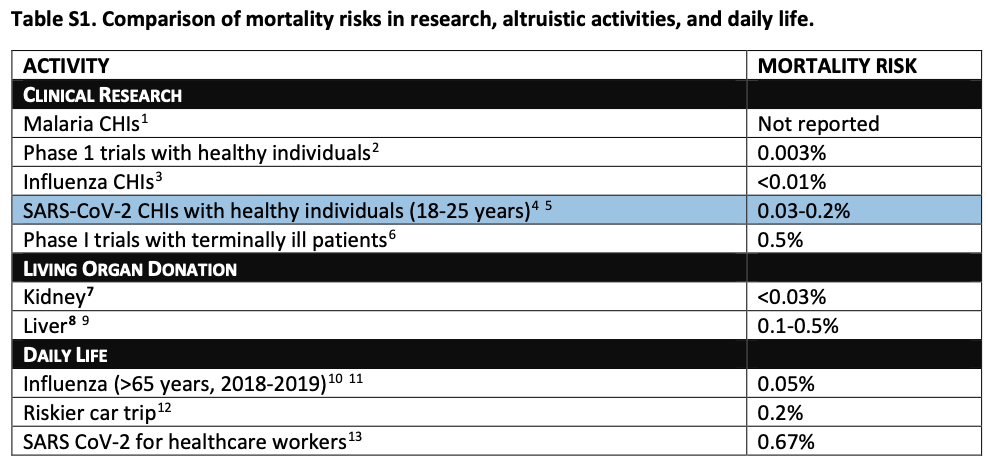
Of course, CHI researchers must obtain robust and ongoing informed consent from the volunteers. The ethicists also observe that "fairness seems to demand offering participants compensation for their time." It certainly does. They propose a compensation of several thousand dollars to each American volunteer.
In an emailed press release, a coronavirus vaccine challenge working group that includes the Harvard epidemiologist Marc Lipsitch and the NYU bioethicist Arthur Caplan agreed with Shah and her colleagues. "The health and economic damage facing the world from the ongoing viral plague, especially in the most vulnerable segments of human society, are staggering," the researchers observe. "Vaccine research that could speed the process to find a safe, efficacious, and practical vaccine would mitigate this ongoing damage, and if done consistent with recognized standards of human participant protection, would be ethical." They correctly conclude that it is therefore "time to promptly fund and initiate the necessary preparations for conducting challenge studies."
