CRISPR Critters: Regulators and the New Gene Revolution in Agriculture
More, better, and safer food - but only if regulators will stay out of the way.

It's CRISPR's world, and we're just living in it. The gene-editing technology, which has been likened to a genetic word processor, allows researchers to make specific changes ranging from tweaking a single DNA base pair to revising whole paragraphs of genetic information. Most of CRISPR's media attention has focused on the vast improvements it could bring to medicine, such as fixing genetic diseases, curing cancer, speeding up vaccine development, and overcoming antibiotic resistance in disease-causing microbes. Less widely reported, but no less momentous, are the dramatic transformations that CRISPR will bring to nearly all aspects of farming. All that is needed for this new green revolution to take off is for regulators to get out of the way.
Thanks to entirely specious fears about the safety of modern biotech crops, regulatory encrustations are significantly slowing the introduction of new genetically modified crop varieties, including some that resist pests, diseases, drought, salt, and heat and are nutritionally improved. In the U.S., the regulation costs of getting a new variety to farmers run about $136 million. As a result, only the bigger companies can afford to develop new crop varieties. The number of seed companies has fallen from scores in the 1980s to just six major companies today.
CRISPR opens an opportunity to avoid the regulatory mistakes of the past. The current biotech rules focus on the fact that plant breeders typically introduce new genes from unrelated species into crops. For example, genes for the insecticidal proteins derived from Bacillus thuringiensis protect corn, soybeans, and cotton from pests. The gene for the EPSPS protein obtained from the soil microbe Agrobacterium confers resistance to the herbicide glyphosate. These genes and the proteins they make are safe for people to eat; yet each new variety incorporating them must undergo regulatory approval before farmers are permitted to plant them.
CRISPR can avoid the introduction of genes from unrelated species into crop plants. In fact, CRISPR's gene-edited crops would be indistinguishable from crops created by means of conventional crossbreeding.
In conventional crossbreeding, researchers identify a useful trait in a landrace or closely related wild variety—say, resistance to mildew. Plant breeders then try to incorporate mildew resistance into high-yielding commercial varieties by crossbreeding. Of course, half of the genes come from each parent, so the first crossbred generations will contain lots of genes from the landrace that mix in undesirable traits that undermine the crop varieties' high-yield potential. So plant breeders have to keep backcrossing for generations and years to isolate the mildew resistance gene into a commercially viable new variety.
CRISPR provides a short cut. Using genomic techniques, plant breeders can identify the exact mildew resistance gene in a landrace and then find the corresponding, less effective version in the commercial variety. The gene in the commercial version can then be edited to perfectly match the more effective landrace gene. The new, gene-edited commercial variety is identical in all relevant respects to a conventionally crossbred mildew resistant commercial variety. In fact, Chinese researchers have reported using gene-editing to create a wheat variety that resists powdery mildew. Since there is no difference between the gene-edited and conventional varieties, there should be no difference how they are regulated.
In February, a group of Chinese, German, and American biotech researchers outlined in Nature Genetics a fairly reasonable regulatory framework for genome-edited crops. They proposed that plant breeders who create gene-edited crops (GECs) should first minimize the risk that they escape from labs and fields during research and development. Then the researchers would have to show that no foreign genes remain in their GEC varieties, document how closely the edited genes match genes found in related species, and make sure there are no off-target edits. If the new GEC variety satisfies these conditions, it should be registered just as any new conventional variety is and regulated no more stringently than any other commercial crop variety. "The opportunities that GECs offer for ensuring global food and nutrition security are at least on the same order as those from GM crops and in many cases are more promising than those from conventional breeding," the researchers note.
An editorial in the same issue of Nature Genetics endorses this proposal. "A distinction must be established, particularly in the public sphere, between 'genetically modified organisms' (GMOs) generated through the transgenic introduction of foreign DNA sequences and 'genome-edited crops' (GECs) generated through precise editing of an organism's native genome," it urges. Given that no one has gotten so much as a cough, sneeze, or bellyache from foods made using current biotech crops, the distinction between GMOs and GECs is not being made for safety reasons, but only as a way try to forestall regulators from applying their already excessive rules to gene-edited crops. So far it seems to be working.
For example, the U.S. Department of Agriculture (USDA) ruled that crops created using other gene-editing techniques do not need its approval. Using its proprietary Rapid Trait Development System (RTDS), the agbio firm Cibus had created a variety of canola that is resistant to its sulfonylurea herbicide. In 2004, the USDA decided—and European regulators agreed—that RTDS is a natural form of targeted mutagenesis, and therefore that crops generated using it do not have to go through an onerous and costly approval process. The USDA is supposed to decide whether it has the authority to regulate CRISPR-generated crops soon.
CRISPR will improve not just crops but livestock. Chinese researchers have used the technique to make muscular goats that grow more hair; American researchers have used it to generate hornless cattle; British researchers have tweaked immune system genes in domestic pigs to match those from warthogs, so that they now resist African swine fever; and South Korean researchers gene-edited pigs to be much meatier. In all cases, no "foreign" DNA was added. It took nearly 20 years for the U.S. Food and Drug Administration to approve the first genetically modified animal for human consumption, the faster growing AquaBounty salmon. Given CRISPR's precision, there is no reason gene-edited livestock should be more highly regulated than conventionally bred cows, goats, and pigs.
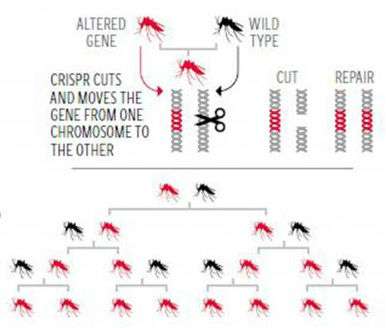
CRISPR could also greatly improve the control of agricultural pests. For example, CRISPR gene-drives could be developed that make insects and weeds once again susceptible to pesticides and herbicides to which they had become resistant. CRISPR could be used to engineer a gene drive that copies itself onto chromosomes that previously were without it. When an organism inherits such a gene drive from a parent, the drive copies itself onto the chromosome from the other parent. In such a way, CRISPR-enabled gene drives could reinstate the earlier susceptible genotypes in pest and weed populations. Restoring an earlier genotype in a pest population should require little regulatory oversight.
Gene drives could also be used to drastically reduce the numbers of a pest species or even force it to go extinct. CRISPR can be used to engineer a gene into the Y chromosome of a targeted pest species that cuts out the X chromosomes from sperm-generating cells, thus ensuring that all progeny are male. Already researchers have created a gene-drive that would greatly reduce the populations of malaria-carrying mosquitoes. Since the effects of Y-drives could extend well beyond farmers' fields and pastures, regulatory scrutiny might be called for when such innovations are released into the ecological open commons.
"For better or for worse, CRISPR–Cas9 is transforming biology," the editors of Nature Genetics observe. "We are now at the dawn of the gene-editing age." In this age, farmers will be able to grow more, better, and safer food while sparing more land for nature—but only if regulators will stay out of the way.

Show Comments (53)