Mail-Order Abortion Pills, Now Officially Authorized by the FDA, Pose an Insoluble Problem for Legislators Who Want To Ban the Procedure
Federal regulators have permanently lifted a requirement that mifepristone be dispensed in person.
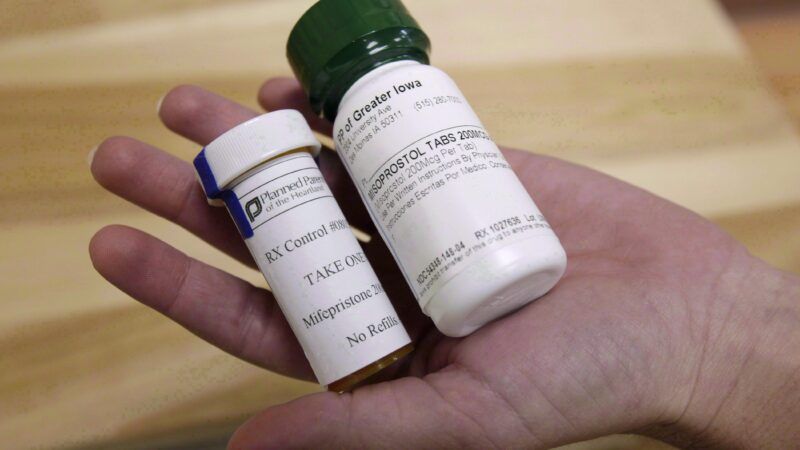
The Food and Drug Administration (FDA) yesterday announced that it is permanently loosening restrictions on the abortion-inducing drug mifepristone, allowing women to receive it by mail after a telemedicine session. The FDA had already used its enforcement discretion to allow that practice for the duration of the COVID-19 pandemic. The new policy preserves the option, which will play an increasingly important role as many states impose new restrictions on abortion, especially if the Supreme Court decides that the Constitution does not protect access to the procedure after all.
The FDA first approved mifepristone, a.k.a. RU-486 and Mifeprex, in 2000. The standard protocol for a medical abortion currently involves a dose of mifepristone, which thins the lining of the uterus by blocking the effects of progesterone, followed one or two days later by a dose of misoprostol, which causes uterine contractions. The FDA has approved the use of that regimen up to 10 weeks into a pregnancy. In 2019, according to the Centers for Disease Control and Prevention (CDC), 79 percent of abortions in the United States were performed at nine weeks or earlier.
The FDA originally required that mifepristone be dispensed in person by a medical provider. An FDA-approved research project launched in 2016, the TelAbortion Study, aimed to assess the safety and efficacy of prescribing the drug based on "a video evaluation over the internet." The program expanded during the pandemic, eventually including 17 states and the District of Columbia. According to a TelAbortion report published last March, covering nearly 1,400 packages of pills mailed from May 2016 through September 2000, "this direct-to-patient telemedicine service was safe, effective, and acceptable, and supports the claim that there is no medical reason for mifepristone to be dispensed in clinics as required by the Food and Drug Administration."
In July 2020, a federal judge in Maryland blocked enforcement of the FDA's general requirement that women visit a medical facility to obtain mifepristone, saying it probably imposed an "undue burden" on abortion access in light of the pandemic. After the U.S. Court of Appeals for the 4th Circuit declined to issue a stay against that preliminary injunction, the Supreme Court in January reinstated the FDA's requirement pending resolution of the agency's appeal.
Three months later, after the incoming Biden administration reevaluated the mifepristone policy, the FDA announced that it would not be enforced "during the COVID-19 PHE" (public health emergency). In an April 12 letter to the American College of Obstetricians and Gynecologists, the lead plaintiff in the case that challenged the FDA rule, Acting FDA Administrator Janet Woodcock said the evidence at that point did "not appear to show increases in serious safety concerns…occurring with medical abortion as a result of modifying the in-person dispensing requirement during the COVID-19 pandemic."
Yesterday the FDA said it had "determined that the data support modification of [mifepristone regulations] to reduce burden on patient access and the health care delivery system and to ensure the benefits of the product outweigh the risks." The new policy still requires that health care providers who approve the use of mifepristone obtain special certification and that patients sign an agreement indicating that they have been informed of the drug's risks. It also adds a certification requirement for pharmacies that supply the drug. But women who want to use mifepristone at home will not be required to visit a doctor or clinic before doing so, regardless of what is happening with COVID-19.
State legislators have responded to the FDA's policy shift by imposing new restrictions on mifepristone. The pro-choice Guttmacher Institute reports that "19 states require the clinician providing a medication abortion to be physically present when the medication is administered, thereby prohibiting the use of telemedicine to prescribe medication for abortion." Seven of those states enacted that requirement this year, while six banned delivery of abortion pills by mail.
Getting around such restrictions is considerably easier than traveling to an out-of-state clinic for a surgical abortion. "The current practice is that women who live in states that don't allow telemedicine for abortion must travel to a state that does — although they don't have to visit a clinic," The New York Times reports. "They may be in any location within that state for their telehealth visit, even a car, and may receive the pills at any address in the state."
There are also less-legal workarounds. In an interview with the Times, Florida State law professor Mary Ziegler, author of Abortion and the Law in America, predicted that "plenty of people" will "try to use [abortion pills] in states where they're illegal without traveling out of state." She "said such efforts might include clearinghouses that would try to allow 'fudging where people's addresses are to receive it' and a 'black market' that might emerge."
That black market already exists. In an October report on the Texas law that prohibits abortion after fetal cardiac activity can be detected (around six weeks of gestation), the Times noted that "some women…cross the border to Mexico, where one of the pills that causes abortions is sold over the counter," adding, "These methods are not technically legal." Websites offering mifepristone and misoprostol purchased in foreign countries are another option, although legally hazardous for the operators.
Now that abortion pills can be legally obtained by mail without an in-person medical appointment in most of the country, local limits will be even easier to evade. If you think states can actually prevent medical abortions, you must be unfamiliar with the war on drugs, which has failed for more than a century to stop Americans from obtaining politically disfavored intoxicants, even when they are illegal in every state.
In 2019, according to the CDC, drug-induced abortions accounted for 42 percent of the total, and that share is likely to rise under the new FDA policy, especially if the Supreme Court allows states to ban or severely restrict surgical abortions, as seems likely. While medical abortions currently are much more common in many other countries, that gap is bound to shrink.
Some countries allow medical abortion after 10 weeks. A 2021 survey of 46 countries or regions in BMJ Sexual & Reproductive Health found that the most permissive limit was nearly 12 weeks. If the FDA adopted that limit, it would cover all but a small percentage of abortions—something like 12 percent, according to the Guttmacher Institute's analysis of CDC data for 2016.
Six years before the Supreme Court ruled that the 14th Amendment guarantees a right to terminate a pregnancy before "viability," a brief story in the Times contemplated the implications of "do-it-yourself abortion." The paper reported that "self-medication may soon be adequate to terminate pregnancies" and asked whether "scientific progress" would "soon make obsolete the centuries-old legal, religious and personal arguments about abortion." That much is beyond the capacity of scientific progress, as more than half a century of continuing debate shows. But a safe and effective DIY option does pose an insurmountable challenge to legislators who hope to eliminate most abortions by force of law.

Show Comments (151)